Tin(IV) fluoride
Tin(IV) fluoride is a chemical compound of tin and fluorine with the chemical formula SnF4 and is a white solid with a melting point above 700 °C.
[1] SnF4 can be prepared by the reaction of tin metal with fluorine gas:[2] However, a passivating metal fluoride layer will be created and the surface will eventually become unreactive.
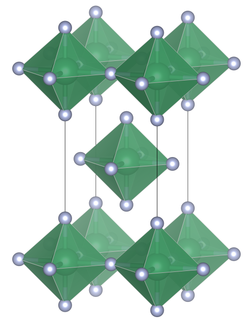
An alternative synthesis is the reaction of SnCl4 with anhydrous hydrogen fluoride:[1] With alkali metal fluorides (e.g. KF) hexafluorostannates are produced (e.g.K2SnF6), which contain the octahedral SnF62− anion.
SnF4 behaves as a Lewis acid and adducts L2·SnF4 and L·SnF4 have been produced.
[1] The structure can also be contrasted with the tetrafluorides of the lighter members of group 14, (CF4, SiF4 and GeF4) which in the solid state form molecular crystals.