Transition metal alkyl complexes
Nucleophilic sources of alkyl ligands include Grignard reagents and organolithium compounds.
Electrophilic alkylation commonly starts with low valence metal complexes.
Such interactions are especially common for complexes of early transition metals in their highest oxidation states.
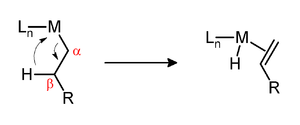
[18] One determinant of the kinetic stability of metal-alkyl complexes is the presence of hydrogen at the position beta to the metal.
These reactions include hydrogenation, hydroformylation, alkene isomerization, and olefin polymerization.