Transition metal imido complex
In coordination chemistry and organometallic chemistry, transition metal imido complexes is a coordination compound containing an imido ligand.
Complexes of the type M=NH are assumed to be intermediates in nitrogen fixation by synthetic catalysts.
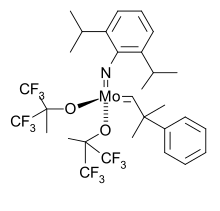
They arise by condensation of amines and metal oxides and metal halides: This approach is illustrated by the conversion of MoO2Cl2 to the diimido derivative MoCl2(NAr)2(dimethoxyethane), precursors to the Schrock carbenes of the type Mo(OR)2(NAr)(CH-t-Bu).
They are however assumed to be intermediates in ammoxidation catalysis, in the Sharpless oxyamination, and in nitrogen fixation.
A molybdenum imido complex appears in a common nitrogen fixation cycle: with the oxidation state of molybdenum varying to accommodate the number bonds from nitrogen.