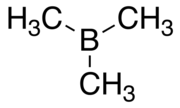
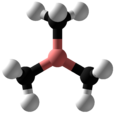
Trimethylborane
[7] Due to its dangerous nature the compound was no longer studied until 1921, when Alfred Stock and Friedrich Zeidler took advantage of the reaction between boron trichloride gas and dimethylzinc.
[8] Although the substance can be prepared using Grignard reagents the output is contaminated by unwanted products from the solvent.
Trimethylborane can be made on a small scale with a 98% yield by reacting trimethylaluminium in hexane with boron tribromide in dibutyl ether as a solvent.
When trimethylborane forms an adduct with trimethylamine, steric repulsion between the methyl groups on the B and N results.
It reacts as a gas with trimethylphosphine to form a solid Lewis salt with a heat of formation of −41 kcal per mol.
[14] The tetramethylborate ion has a negative charge and is isoelectronic with neopentane, tetramethylsilane, and the tetramethylammonium cation.