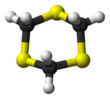
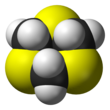
1,3,5-Trithiane
It consists of a six-membered ring with alternating methylene bridges and thioether groups.
[2] Trithiane is a building block molecule in organic synthesis, being a masked source of formaldehyde.
In one application, it is deprotonated with organolithium reagents to give the lithium derivative, which can be alkylated.
For example, chlorination in the presence of water affords the chloromethyl sulfonyl chloride:[4] Trithiane is the parent of a class of heterocycles called trithianes, that formally result from substitution of various monovalent groups for one or more of the hydrogen atoms.
Alternatively 1,3,5-trithiane can be deprotonated and alkylated to afford (SCH2)n(SCHR)3-n.[5] The conformation of trithianes has been well investigated.