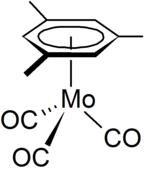Organomolybdenum chemistry
The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.
[4] CO-free Mo(0) compounds tend to be more reducing and kinetically labile than the carbonyl complexes.
[7] One large collection of compounds have the formula (C5R5)Mo(CO)3X, derived from cyclopentadienylmolybdenum tricarbonyl dimer (X = halide, hydride, alkyl).
[5] Oxo and imide (RN=) ligands are found in several high oxidation state organomolybdenum compounds.
[9] Schrock's Mo-based olefin metathesis catalysts feature molybdenum(VI) centers supported by alkoxide, alkylidene, and imido ligands.
[10] Molybdenum neopentylidyne complexes endowed with sterically demanding phenolates or branched fluorinated alkoxides catalyze alkyne metathesis.