Photo-oxidation of polymers
For example, plastic building components like doors, window frames and gutters are expected to last for decades, requiring the use of advanced UV-polymer stabilizers.
Polyolefins such as polyethylene and polypropylene are susceptible to photo-oxidation and around 70% of light stabilizers produced world-wide are used in their protection, despite them representing only around 50% of global plastic production.
[3] The bulk of the polymer is therefore photo-inert and degradation is instead attributed to the presence of various impurities, which are introduced during the manufacturing or processing stages.
[4] The organic hydroperoxide and carbonyl groups are able to absorb UV light above 290 nm whereupon they undergo photolysis to generate radicals.
For polystyrene the complete mechanism of photo-oxidation is still a matter of debate, as different pathways may operate concurrently[20] and vary according to the wavelength of the incident light.
acetophenone) also absorb light in the near ultraviolet range (300 to 400 nm), forming excited ketones able to abstract hydrogen atoms directly from the polymer.
The propagation steps are essentially identical to those seen for polyolefins; with oxidation, hydrogen abstraction and photolysis leading to beta scission reactions and increasing numbers of radicals.
These steps account for the majority of chain-breaking, however in a minor pathway the hydroperoxide reacts directly with polymer to form a ketone group (acetophenone) and a terminal alkene without the formation of additional radicals.
The initiation of photo-oxidation is instead caused by various irregularities in the polymer chain, such as structural defects[26][27] as well as hydroperoxides, carbonyl groups, and double bonds.
[29][31][32] Propagation steps involve the hydroperoxyl radical, which can abstract hydrogen from both hydrocarbon (-CH2-) and organochloride (-CH2Cl-) sites in the polymer at comparable rates.
When the polyenes contain at least eight conjugated double bonds they become coloured, leading to yellowing and eventual browning of the material.
This is off-set slightly by longer polyenes being photobleached with atmospheric oxygen,[33] however PVC does eventually discolour unless polymer stabilisers are present.
Reactions at organochloride sites proceed via the usual hydroperoxyl and hydroperoxide before photolysis yields the α-chloro-alkoxyl radical.
[1][35][36] Despite this PET has better resistance to photo-oxidation than other commodity plastics, this is due to a poor quantum yield or the absorption.
[37] The degradation chemistry is complicated due to simultaneous photodissociation (i.e. not involving oxygen) and photo-oxidation reactions of both the aromatic and aliphatic parts of the molecule.
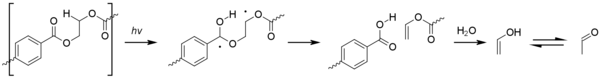
The type I reaction dominates, which cause chain scission at the carbonyl unit to give a range of products.
[1][38] Type II Norrish reactions are less common but give rise to acetaldehyde by way of vinyl alcohol esters.
The photo-oxidation process at aliphatic sites is similar to that seen for polyolefins, with the formation of hydroperoxide species eventually leading to beta-scission of the polymer chain.
By comparison the dependence of degradation rate on UV exposure and the availability of oxygen is broadly linear.
Mechanical stress can effect the rate of photo-oxidation[42] and may also accelerate the physical breakup of plastic objects.
[44] Fillers such as carbon black can screen out UV light, effectively stabilisers the polymer, whereas flame retardants tend to cause increased levels of photo-oxidation.
Fe complexes increase the rate of photooxidation by promoting the homolysis of hydroperoxides via Fenton reactions.
The use of such additives has been controversial due to concerns that treated plastics do not fully biodegrade and instead result in the accelerated formation of microplastics.
OXO-biodegradation additives were banned in the EU in 2019[50] UV attack by sunlight can be ameliorated or prevented by adding anti-UV polymer stabilizers, usually prior to shaping the product by injection moulding.
Through weather testing, the impact of photooxidative processes on the mechanical properties and lifetimes of polymer samples can be determined.
Stress is usually applied until the material fractures, and from this stress–strain curve, mechanical properties such as the Young's modulus can be determined.
[56] Mathematical models can also be created to predict the change in mass of a polymer sample over the weathering process.
Thus, a model for the dependence of degradation on surface area can be made by assuming that the rate of change in mass
is the density and kd is known as the specific surface degradation rate (SSDR), which changes depending on the polymer sample's chemical composition and weathering environment.
In the example shown at left, carbonyl groups were easily detected by IR spectroscopy from a cast thin film.



PP: polypropylene , PE: polyethylene , PVC: Polyvinyl chloride , PS: Polystyrene , PET: Polyethylene terephthalate