Mendelevium
All known isotopes of mendelevium have short half-lives; there are currently no uses for it outside basic scientific research, and only small amounts are produced.
It was first synthesized by Albert Ghiorso, Glenn T. Seaborg, Gregory Robert Choppin, Bernard G. Harvey, and team leader Stanley G. Thompson in early 1955 at the University of California, Berkeley.
[5] This discovery was part of a program, begun in 1952, that irradiated plutonium with neutrons to transmute it into heavier actinides.
[6] This method was necessary as the previous method used to synthesize transuranic elements, neutron capture, could not work because of a lack of known beta decaying isotopes of fermium that would produce isotopes of the next element, mendelevium, and also due to the very short half-life to spontaneous fission of 258Fm that thus constituted a hard limit to the success of the neutron capture process.
The number of atoms that would be produced would be approximately equal to the product of the number of atoms of target material, the target's cross section, the ion beam intensity, and the time of bombardment; this last factor was related to the half-life of the product when bombarding for a time on the order of its half-life.
[5] The target material, einsteinium-253, could be produced readily from irradiating plutonium: one year of irradiation would give a billion atoms, and its three-week half-life meant that the element 101 experiments could be conducted in one week after the produced einsteinium was separated and purified to make the target.
However, it was necessary to upgrade the cyclotron to obtain the needed intensity of 1014 alpha particles per second; Seaborg applied for the necessary funds.
[6] While Seaborg applied for funding, Harvey worked on the einsteinium target, while Thomson and Choppin focused on methods for chemical isolation.
This technique gave a very high yield, which was absolutely necessary when working with such a rare and valuable product as the einsteinium target material.
[6][8] The resultant drops entered a test tube, which Choppin and Ghiorso took in a car to get to the Radiation Laboratory as soon as possible.
[6] Additional analysis and further experimentation showed the produced mendelevium isotope to have mass 256 and to decay by electron capture to fermium-256 with a half-life of 157.6 minutes.
[4] We thought it fitting that there be an element named for the Russian chemist Dmitri Mendeleev, who had developed the periodic table.
But in the middle of the Cold War, naming an element for a Russian was a somewhat bold gesture that did not sit well with some American critics.
Because this discovery came during the Cold War, Seaborg had to request permission of the government of the United States to propose that the element be named for a Russian, but it was granted.
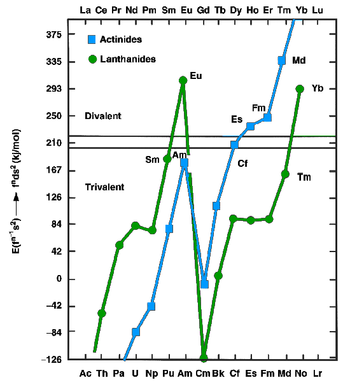
[14][15] The conclusion was that the increased binding energy of the [Rn]5f126d17s2 configuration over the [Rn]5f137s2 configuration for mendelevium was not enough to compensate for the energy needed to promote one 5f electron to 6d, as is true also for the very late actinides: thus einsteinium, fermium, mendelevium, and nobelium were expected to be divalent metals.
[13] Like the other divalent late actinides (except the once again trivalent lawrencium), metallic mendelevium should assume a face-centered cubic crystal structure.
[18] Before mendelevium's discovery, Seaborg and Katz predicted that it should be predominantly trivalent in aqueous solution and hence should behave similarly to other tripositive lanthanides and actinides.
After the synthesis of mendelevium in 1955, these predictions were confirmed, first in the observation at its discovery that it eluted just after fermium in the trivalent actinide elution sequence from a cation-exchange column of resin, and later the 1967 observation that mendelevium could form insoluble hydroxides and fluorides that coprecipitated with trivalent lanthanide salts.
[4] Nevertheless, the shorter-lived 256Md (half-life 1.295 hours) is more often used in chemical experimentation because it can be produced in larger quantities from alpha particle irradiation of einsteinium.
[4][23] Experiments and predictions suggest that the half-lives will then decrease, apart from 260Md with a half-life of 27.8 days,[4][23] as spontaneous fission becomes the dominant decay mode[4] due to the mutual repulsion of the protons posing a limit to the island of relative stability of long-lived nuclei in the actinide series.
[24] In addition, mendelevium is the element with the highest atomic number that has a known isotope with a half-life longer than one day.
[25] Using a long capillary tube, and including potassium chloride aerosols in the helium gas, the mendelevium atoms can be transported over tens of meters to be chemically analyzed and have their quantity determined.
[25] Mendelevium can finally be separated from the other trivalent actinides using selective elution from a cation-exchange resin column, the eluant being ammonia α-HIB.
Then, 50 mg of chromium is added to the mendelevium to reduce it to the +2 state in 0.1 M hydrochloric acid with zinc or mercury.
[25] The solvent extraction then proceeds, and while the trivalent and tetravalent lanthanides and actinides remain on the column, mendelevium(II) does not and stays in the hydrochloric acid.
[25] Though few people come in contact with mendelevium, the International Commission on Radiological Protection has set annual exposure limits for the most stable isotope.