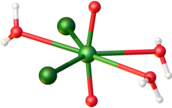
Uranyl chloride
[3] The anhydrous material can be obtained by the reaction of oxygen with uranium tetrachloride: In terms of structures, all three of these compounds feature the uranyl center (trans-UO22+) bound to five additional ligands, which can include (bridging) chloride, water, or another uranyl oxygen.
[6] The company Indian Rare Earths Limited (IREL) has developed a process to extract uranium from the Western and Eastern coastal dune sands of India.
After pre-processing with high-intensity magnetic separators and fine grinding, the mineral sands (known as monazite), are digested with caustic soda at about 120 °C (248 °F) and water.
The solution is subjected to liquid–liquid extraction with dual solvent systems to produce uranyl chloride and thorium oxalate.
The crude uranyl chloride solution is subsequently refined to nuclear grade ammonium diuranate by a purification process involving precipitation and solvent extraction in a nitrate media.