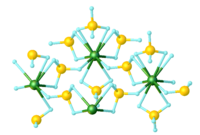
Uranium borohydride
Two polymeric forms are known, as well as a monomeric derivative that exists in the gas phase.
Because the polymers convert to the gaseous form at mild temperatures, uranium borohydride once attracted much attention.
[2] Gaseous features a monomeric 12-coordinate uranium, with four κ3-BH4− ligands, which envelop the metal, conferring volatility.
[3] This compound was first prepared by treating uranium tetrafluoride with aluminium borohydride:[1] It may also be prepared by the solid-state reaction of uranium tetrachloride with lithium borohydride:[1] Although solid U(BH4)4 is a polymer, it undergoes cracking, converting to the monomer.
However, by the time the synthesis method was finalized, the problems related to uranium hexafluoride were solved.