Uranium compounds
Although uranium is a radioactive actinide, its compounds are well studied due to its long half-life and its applications.
Uranium content is usually referenced to U3O8, which dates to the days of the Manhattan Project when U3O8 was used as an analytical chemistry reporting standard.
[3] Both oxide forms are solids that have low solubility in water and are relatively stable over a wide range of environmental conditions.
Triuranium octoxide is (depending on conditions) the most stable compound of uranium and is the form most commonly found in nature.
Because of their stability, uranium oxides are generally considered the preferred chemical form for storage or disposal.
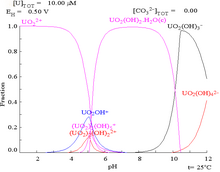
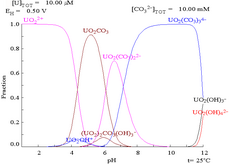
The most common ionic forms are U3+ (brown-red), U4+ (green), UO+2 (unstable), and UO2+2 (yellow), for U(III), U(IV), U(V), and U(VI), respectively.
UO2+2 also forms complexes with various organic chelating agents, the most commonly encountered of which is uranyl acetate.
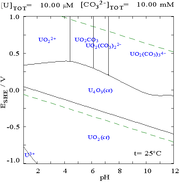
This is because a U(VI) cation is able to bind two terminal oxides and three or more carbonates to form anionic complexes.
Under the right conditions of temperature and pressure, the reaction of solid UF4 with gaseous uranium hexafluoride (UF6) can form the intermediate fluorides of U2F9, U4F17, and UF5.
[6] At room temperatures, UF6 has a high vapor pressure, making it useful in the gaseous diffusion process to separate the rare uranium-235 from the common uranium-238 isotope.