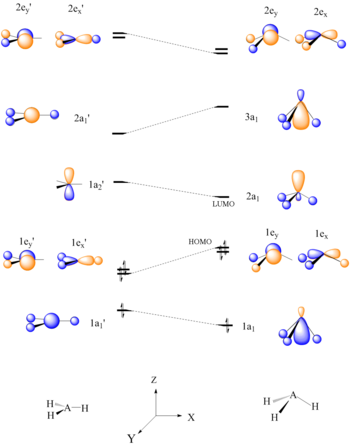
Walsh diagram
If a particular structural change does not perturb the HOMO, the closest occupied molecular orbital governs the preference for geometrical orientation.
Specifically, Walsh calculated and explained the effect of changes in the shape of a molecule on the energy of molecular orbitals.
"[15] In his publications, Walsh showed through multiple examples that the geometry adopted by a molecule in its ground state primarily depends on the number of its valence electrons.
[17][18] However, Mulliken was unable to explain the reasons for the rises and falls of certain curves with increases in angle, thus Walsh claimed "his diagram was either empirical or based upon unpublished computations.
"[5] Walsh originally constructed his diagrams by plotting what he described as "orbital binding energies" versus bond angles.
What Walsh was actually describing by this term is unclear; some believe he was in fact referring to ionization potentials, however this remains a topic of debate.
Typically, core orbitals (1s for B, C, N, O, F, and Ne) are excluded from Walsh diagrams because they are so low in energy that they do not experience a significant change by variations in bond angle.
The single-point computation for each geometry can then be plotted versus bond angle to produce the representative Walsh diagram.