Zinc
[18] Brass, an alloy of copper and zinc in various proportions, was used as early as the third millennium BC in the Aegean area and the region which currently includes Iraq, the United Arab Emirates, Kalmykia, Turkmenistan and Georgia.
Other metals long known to form binary alloys with zinc are aluminium, antimony, bismuth, gold, iron, lead, mercury, silver, tin, magnesium, cobalt, nickel, tellurium, and sodium.
[35][39][40] The most recent estimate of reserve base for zinc (meets specified minimum physical criteria related to current mining and production practices) was made in 2009 and calculated to be roughly 480 Mt.
[41] Zinc reserves, on the other hand, are geologically identified ore bodies whose suitability for recovery is economically based (location, grade, quality, and quantity) at the time of determination.
Consequently some of the equivalent salts have the same crystal structure,[57] and in other circumstances where ionic radius is a determining factor, the chemistry of zinc has much in common with that of magnesium.
[84] The Rasaratna Samuccaya, written in approximately the 13th century AD, mentions two types of zinc-containing ores: one used for metal extraction and another used for medicinal purposes.
[85] Zinc was distinctly recognized as a metal under the designation of Yasada or Jasada in the medical Lexicon ascribed to the Hindu king Madanapala (of Taka dynasty) and written about the year 1374.
Some alchemists called this zinc oxide lana philosophica, Latin for "philosopher's wool", because it collected in wooly tufts, whereas others thought it looked like white snow and named it nix album.
[87][89] The word is probably derived from the German zinke, and supposedly meant "tooth-like, pointed or jagged" (metallic zinc crystals have a needle-like appearance).
[34] German metallurgist Andreas Libavius received a quantity of what he called "calay" (from the Malay or Hindi word for tin) originating from Malabar off a cargo ship captured from the Portuguese in the year 1596.
[97] Postlewayt's Universal Dictionary, a contemporary source giving technological information in Europe, did not mention zinc before 1751 but the element was studied before then.
[118] The dumps of the past mining operations leach zinc and cadmium, and the sediments of the Geul River contain non-trivial amounts of metals.
Levels of zinc in excess of 500 ppm in soil interfere with the ability of plants to absorb other essential metals, such as iron and manganese.
Galvanization is used on chain-link fencing, guard rails, suspension bridges, lightposts, metal roofs, heat exchangers, and car bodies.
[127] The relative reactivity of zinc and its ability to attract oxidation to itself makes it an efficient sacrificial anode in cathodic protection (CP).
[126] Other widely used zinc alloys include nickel silver, typewriter metal, soft and aluminium solder, and commercial bronze.
[154] Zinc sheet metal is used as a durable covering for roofs, walls, and countertops, the last often seen in bistros and oyster bars, and is known for the rustic look imparted by its surface oxidation in use to a blue-gray patina and susceptibility to scratching.
[155][156][157][158] 64Zn, the most abundant isotope of zinc, is very susceptible to neutron activation, being transmuted into the highly radioactive 65Zn, which has a half-life of 244 days and produces intense gamma radiation.
Quantitative results (yield and enantiomeric excess) obtained with chiral zinc catalysts can be comparable to those achieved with palladium, ruthenium, iridium and others.
[9][203][10] Dysregulation of zinc homeostasis in the central nervous system that results in excessive synaptic zinc concentrations is believed to induce neurotoxicity through mitochondrial oxidative stress (e.g., by disrupting certain enzymes involved in the electron transport chain, including complex I, complex III, and α-ketoglutarate dehydrogenase), the dysregulation of calcium homeostasis, glutamatergic neuronal excitotoxicity, and interference with intraneuronal signal transduction.
[212] The non-related β-carbonic anhydrase is required in plants for leaf formation, the synthesis of indole acetic acid (auxin) and alcoholic fermentation.
A coordinate covalent bond is formed between the terminal peptide and a C=O group attached to zinc, which gives the carbon a positive charge.
[224] The U.S. Institute of Medicine (IOM) updated Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for zinc in 2001.
[231] For fortification, however, a 2003 review recommended cereals (containing zinc oxide) as a cheap, stable source that is as easily absorbed as the more expensive forms.
[232] A 2005 study found that various compounds of zinc, including oxide and sulfate, did not show statistically significant differences in absorption when added as fortificants to maize tortillas.
[234] Zinc supplements help prevent disease and reduce mortality, especially among children with low birth weight or stunted growth.
[240] Major plant sources of zinc include cooked dried beans, sea vegetables, fortified cereals, soy foods, nuts, peas, and seeds.
[241] Species of Calluna, Erica and Vaccinium can grow in zinc-metalliferous soils, because translocation of toxic ions is prevented by the action of ericoid mycorrhizal fungi.
[250] Recent research suggests that the topical antimicrobial zinc pyrithione is a potent heat shock response inducer that may impair genomic integrity with induction of PARP-dependent energy crisis in cultured human keratinocytes and melanocytes.
One reported case of chronic ingestion of 425 pennies (over 1 kg of zinc) resulted in death due to gastrointestinal bacterial and fungal sepsis.



3 CO
2 )
2





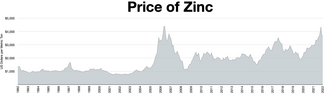
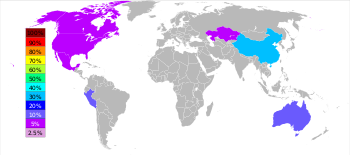
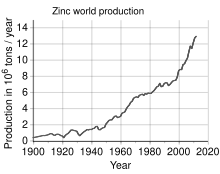

27°57′17″S 016°46′00″E / 27.95472°S 16.76667°E

27°49′09″S 016°36′28″E / 27.81917°S 16.60778°E