Adiabatic electron transfer
[1] [2] Electron transfer during a collision between an oxidant and a reductant occurs adiabatically on a continuous potential energy surface.
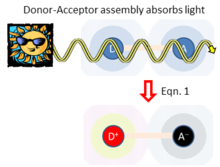
labelled D (for “donor”) and A (for “acceptor”) become a distance R apart, either through collisions, covalent bonding, location in a material, protein or polymer structure, etc.
Adiabatic electron-transfer theory stresses that intricately coupled to such charge transfer is the ability of any D-A system to absorb or emit light.
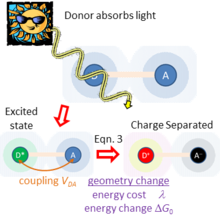
Figure 2 sketches what happens if light is absorbed by just one of the chemical species, taken to be the charge donor.
[5] The inverse of this process is also used to make organic light-emitting diodes (OLEDs).
), Hush showed[2] that the rate of light absorption (and hence charge separation) is given from the Einstein equation by This theory explained[2] how Prussian blue absorbes light, creating[6] [7][8] [9][10] the field of intervalence charge transfer spectroscopy.
Adiabatic electron transfer is also relevant to the Robin-Day classification system, which codifies types of mixed valence compounds.
is not small: charge is not localized on just one chemical species but is shared quantum mechanically between two Ru centers, presenting classically forbidden half-integral valence states.
[13] that the critical requirement for this phenomenon is Adiabatic electron-transfer theory stems from London's approach to charge-transfer and indeed general chemical reactions[14] applied by Hush using parabolic potential-energy surfaces.
[15][16] Hush himself has carried out many theoretical and experimental studies of mixed valence complexes and long range electron transfer in biological systems.
Hush's quantum-electronic adiabatic approach to electron transfer was unique; directly connecting with the Quantum Chemistry concepts of Mulliken, it forms the basis of all modern computational approaches to modeling electron transfer.
It also leads seamlessly[21] to understanding electron-transfer transition-state spectroscopy pioneered by Zewail.
When electron transfer occurs during collisions of the D and A species, the coupling is typically large and the “adiabatic” limit applies in which rate constants are given by transition state theory.
[4] In biological applications, however, as well as some organic conductors and other device materials, R is externally constrained and so the coupling set at low or high values.
In the weak-coupling (“non-adiabatic”) limit, the activation energy for electron transfer is given by the expression derived independently by Kubo and Toyozawa[22] and by Hush.
[16] Using adiabatic electron-transfer theory,[23] in this limit Levich and Dogonadze then determined the electron-tunneling probability to express the rate constant for thermal reactions as[24] This approach is widely applicable to long-range ground-state intramolecular electron transfer, electron transfer in biology, and electron transfer in conducting materials.
It also typically controls the rate of charge separation in the excited-state photochemical application described in Figure 2 and related problems.
In that work,[25] he also derived the standard expression for the solvent contribution to the reorganization energy, making the theory more applicable to practical problems.
Use of this solvation description (instead[4] of the form that Hush originally proposed[16]) in approaches spanning the adiabatic and non-adiabatic limits is often termed “Marcus-Hush Theory”.
Adiabatic electron-transfer theory is also widely applied [2] in Molecular Electronics.