Ammoxidation
In organic chemistry, ammoxidation is a process for the production of nitriles (R−C≡N) using ammonia (NH3) and oxygen (O2).
It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio.
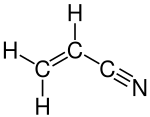
Several million tons of acrylonitrile are produced in this way annually:[3][4] Ammoxidation of alkenes exploits the weak C-H bonds that are located in the allylic position of unsaturated hydrocarbons.
For the production of acrylonitrile, byproducts include hydrogen cyanide, acrolein, and the solvent acetonitrile.
The nitrile process is used industrially to produce nitriles from fatty acids: Hydrogen cyanide is prepared by an ammoxidation-like reaction of methane, the Andrussov oxidation: