Anthranilic acid
[10] Above 81 °C (178 °F; 354 K), it converts to an orthorhombic form with space group Pbca, which is not triboluminescent; a non-triboluminescent monoclinic phase with similar structure is also known.
[10] In 1840-1841, Carl Julius Fritzsche was able to extract and crystallize two acids from the products of reaction of indigo dye with caustic potash, which he called chrysanilic and anthranilic acids after their colors before purification (golden yellow and black respectively) and the plant anil (Indigofera suffruticosa).
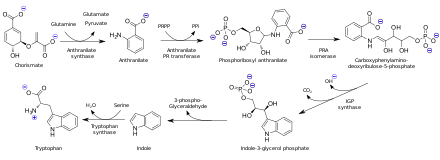
[16] In the era when indigo dye was obtained from plants, it was degraded to give anthranilic acid.
This cation can be used to generate benzyne,[20] dimerized to give diphenic acid,[21] or undergo diazonium coupling reactions such as in the synthesis of methyl red.
[23] Chlorination of anthranilic acid gives the 2,4-dichloro derivative, which can undergo reductive coupling to form a biaryl compound.
[24] It is also a DEA List I Chemical because of its use in making the now-widely outlawed euphoric sedative drug methaqualone (Quaalude, Mandrax).