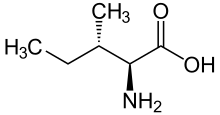
Isoleucine
It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms).
In mammals acetyl-CoA cannot be converted to carbohydrate but can be either fed into the TCA cycle by condensing with oxaloacetate to form citrate or used in the synthesis of ketone bodies (hence ketogenic) or fatty acids.
[7][8] Reduced dietary levels of isoleucine are required for the beneficial metabolic effects of a low protein diet.
[8] The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine has set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002.
One example is maple syrup urine disease (MSUD), a disorder that leaves people unable to breakdown isoleucine, valine, and leucine.
[14] People with MSUD manage their disease by a reduced intake of all three of those amino acids alongside drugs that help excrete built-up toxins.
[5] Foods that have high amounts of isoleucine include eggs, soy protein, seaweed, turkey, chicken, lamb, cheese, and fish.
[17] German chemist Felix Ehrlich discovered isoleucine while studying the composition of beet-sugar molasses 1903.