Beta-ketoacyl-ACP synthase
In molecular biology, Beta-ketoacyl-ACP synthase EC 2.3.1.41, is an enzyme involved in fatty acid synthesis.
In animals, fungi, and lower eukaryotes, Beta-ketoacyl-ACP synthases make up one of the catalytic domains of larger multifunctional proteins (Type I), whereas in most prokaryotes as well as in plastids and mitochondria, Beta-ketoacyl-ACP synthases are separate protein chains that usually form dimers (Type II).
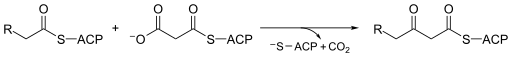
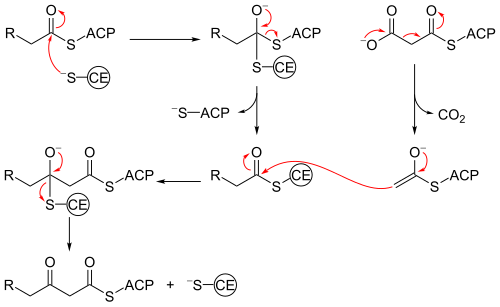
[1][2] Beta-ketoacyl-ACP synthase III, perhaps the most well known of this family of enzymes, catalyzes a Claisen condensation between acetyl CoA and malonyl ACP.
Type I FAS catalyzes all the reactions necessary to create palmitic acid, which is a necessary function in animals for metabolic processes, one of which includes the formation of sphingosines.
[1] Beta-ketoacyl-ACP synthase is found as a component of a number of enzymatic systems, including fatty acid synthetase (FAS); the multi-functional 6-methysalicylic acid synthase (MSAS) from Penicillium patulum,[3] which is involved in the biosynthesis of a polyketide antibiotic; polyketide antibiotic synthase enzyme systems; Emericella nidulans multifunctional protein Wa, which is involved in the biosynthesis of conidial green pigment; Rhizobium nodulation protein nodE, which probably acts as a beta-ketoacyl synthase in the synthesis of the nodulation Nod factor fatty acyl chain; and yeast mitochondrial protein CEM1.
[1] The presence of similar ketoacyl synthases present in all living organisms point to a common ancestor.
[6] In synthase II, each subunit consists of a five-stranded beta pleated sheet surrounded by multiple alpha helices, shown in the image on the left.
[9] The main function of beta-ketoacyl-ACP synthase is to produce fatty acids of various lengths for use by the organism.
Further, palmitic acid, which is created by the beta-ketoacyl-synthases on type I FAS, is used in a number of biological capacities.
FabH catalyzes the quintessential ketoacyl synthase reaction with malonyl ACP and acetyl CoA.
[11] By studying Yersinia pestis, which causes bubonic, pneumonic, and septicaemic plagues, researchers have shown that FabB, FabF, and FabH can theoretically all be inhibited by the same drug due to similarities in their binding sites.
Using E. coli as the organism of choice, engineers have replaced the endogenous FabH domain on FAS, which favors unbranched chains, with FabH versions that favor branching due to their high substrate specificity for branched acyl-ACPs.