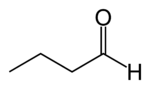
Butyraldehyde
The dominant technology involves the use of rhodium catalysts derived from the water-soluble ligand tppts.
An aqueous solution of the rhodium catalyst converts the propylene to the aldehyde, which forms a lighter (less dense) immiscible phase.
Important reactions include hydrogenation to the alcohol, oxidation to the acid, and base-catalyzed condensation.
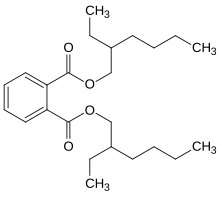
This unsaturated aldehyde is then partially hydrogenated to form 2-ethylhexanal, a precursor to plasticizers such as bis(2-ethylhexyl) phthalate.
[4] Butyraldehyde is a component in the two-step synthesis of trimethylolpropane, which is used for the production of alkyd resins.