CCR5 receptor antagonist
The C-C motif chemokine receptor CCR5 is involved in the process by which HIV, the virus that causes AIDS, enters cells.
The life cycle of the HIV presents potential targets for drug therapy, one of them being the viral entry pathway.
[2] The trigger for the discovery of the CCR5 antagonists was the observation that a small percentage of high-risk populations showed either resistance or delayed development of the disease.
Before the discovery of CCR5's role in HIV infection, many pharmaceutical companies had already built a substantial collection of compounds that target GPCRs.
[citation needed] A significant problem was the affinity of available screening hits for the hERG ion channel;[citation needed] inhibition of hERG leads to QT interval prolongation, which can increase the risk of developing fatal ventricular arrhythmias.
It is being investigated as a potential therapy in the treatment of HIV infection,[18] graft versus host disease (NCT02737306) and metastatic cancer (NCT03838367).
[20][21] In February 2018 CytoDyn Inc reported that the primary endpoint has been achieved in the PRO 140 pivotal combination therapy trial in HIV infection.
In May 2007, results from the phase I clinical trial of the drug demonstrated "potent, rapid, prolonged, dose-dependent, highly significant antiviral activity" for leronlimab.
Participants in the highest-dosing group received 5 milligrams per kilogram and showed an average viral load decrease of -1.83 log10.
[31] The report discloses that a single 350 mg subcutaneous injection of PRO 140 resulted in a HIV-1 RNA viral load reduction greater than 0.5log or 68% within one week compared with those who received a placebo.
In March 2019, CytoDyn filed with the US FDA the first part of the BLA for leronlimab (PRO140) as a combination therapy with HAART in HIV.
On November 23, 2018, CytoDyn received FDA approval of its IND submission and allowed to initiate a Phase Ib/II clinical trial for metastatic triple-negative breast cancer (mTNBC) patients.
In May 2019, the U.S. Food and Drug Administration (FDA) granted fast track designation for leronlimab for use in combination with carboplatin for the treatment of patients with CCR5-positive mTNBC.
Simultaneously, the Phase Ib/II trial for treatment-naïve mTNBC patients is active and anticipates top line data in 2020.
If successful, the data from treatment-naïve mTNBC patients could serve as the basis for potentially seeking accelerated US FDA approval.
Unfortunately, despite the promising preclinical and early clinical results, some severe liver toxicity was observed in the treatment of naïve and treatment-experienced patients that led to the discontinuation in further development of aplaviroc.
The lead compound contained a piperazine scaffold and was a potent muscarinic acetylcholine receptor (M2) antagonist with modest CCR5 activity.
[36][37] Further reconstruction led to the development of the final compound vicriviroc, when Schering discovered that the pyridyl N-oxide on the intermediate could be replaced by 4,6-dimethylpyrimidine carboxamide.
Their screening resulted in a compound that presented weak affinity and no antiviral activity but represented a good starting point for further optimization.
However, the problem with the CYP2D6 activity of the compound was still unacceptable so they had to perform further SAR optimization that determined that the [3.2.1]-azabicycloamine (tropane) could replace the aminopiperidine moiety.
Compound 5 shows an analogue that they synthesized which contained an oxygen bridgehead in the tropane ring; however, that reconstruction did not have an effect on the hERG affinity.
Maraviroc preserved excellent antiviral activity, whilst demonstrating no significant hERG binding affinity.
The lack of hERG binding affinity was predicted to be because of the large size of the cyclohexyl group and the high polarity of the fluoro substituents.
[4][7] The predictive pharmacophore model was developed for a large series of piperidine- and piperazine-based CCR5 antagonists by Schering-Plough Research Institute.
It is possible to use the model as a tool in virtual screening for new small molecular CCR5 antagonists and also to predict biological activities of compounds prior to undertaking their costly synthesis.
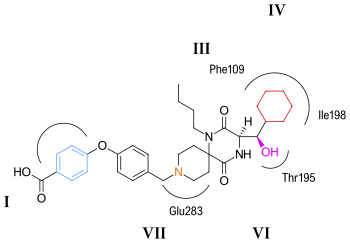
The researchers predict that the butyl group of aplaviroc is buried within the helical bundle through strong hydrophobic interaction with multiple aromatic residues of the CCR5 receptor.
[46] Aplaviroc has a unique feature of preserving two of the natural chemokine protein ligands binding to CCR5 and subsequent activation, whereas maraviroc and the other antagonists almost fully block chemokine-CCR5 interactions.
This kind of interference is so far considered to be safe, and individuals that naturally lack CCR5 do not show any obvious health problems.
Combination of the spiropiperidine template with pharmacophore elements from both aplaviroc, and Schering's CCR5 antagonist program, led to the initial lead compound in this series.
[50] Not only small molecules but also proteins delivered by gene therapy have been suggested to ablate CCR5 function,[51] an approach that has also been employed for other HIV targets.