Carbohydrate synthesis
The generation of carbohydrate structures usually involves linking monosaccharides or oligosaccharides through glycosidic bonds, a process called glycosylation.
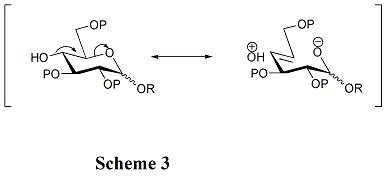
Therefore, it is important to construct glycosidic linkages that have optimum molecular geometry (stereoselectivity) and the stable bond (regioselectivity) at the reaction site (anomeric centre).
Monosacharides (also called "simple sugars") are the simplest single units of any carbohydrate; the most common monosaccharides are five and six carbon compounds such as glucose, fructose, and galactose.
[2] Complex carbohydrates are combinations of monosaccarides linked together through connections called glycosidic bonds, the product of these linkages can be further categorized according to their size.
In nature, monosaccharides are synthesized biologically from raw materials through the processes of photosynthesis in plants and certain prokaryotes, or by gluconeogenesis in animals.
[3] So far, there has not been a unified synthetic strategy of consistent oligosaccharide production because of the nuances in the anomeric effects of monomers and the complexity in the carbohydrate structures.
[12] This process only begins when glycogen storages are near depletion due to the higher ATP cost of metabolising proteins into amino acids.
[12] Conversely, plants undergo the Calvin Cycle to photosynthesize glucose-3-phosphate from CO2 and H2O in the presence of light; the phosphate is quickly hydrolyzed into glucose.
The number of monosaccharides, ring size, the different anomeric stereochemistry, and the existence of the branched-chain sugars all contribute to the amazing complexity of the oligosaccharide structures.
Furthermore, in the preparation of 1, 2-trans glycosidic linkage, using thioglycosides and imidates can promote the rearrangement of the orthoester byproducts, since the reaction mixtures are acidic enough.
Typically, when non-participating groups on O-2 position, 1, 2-cis-β-linkage can be achieved either by using the historically important halide ion methods, or by using 2-O-alkylated glycosyl donors, commonly thioglycosides or trichloroacetimidates, in nonpolar solvents.
However, the method introduced by David Crich (Scheme 4), with 4,6-benzylidene protection a prerequisite and anomeric alpha triflate a key intermediate leaves this problem essentially solved.