Nitrogen
The name nitrogène was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790 when it was found that nitrogen was present in nitric acid and nitrates.
Many drugs are mimics or prodrugs of natural nitrogen-containing signal molecules: for example, the organic nitrates nitroglycerin and nitroprusside control blood pressure by metabolising into nitric oxide.
The mixture of nitric and hydrochloric acids was known as aqua regia (royal water), celebrated for its ability to dissolve gold, the king of metals.
French chemist Antoine Lavoisier referred to nitrogen gas as "mephitic air" or azote, from the Greek word άζωτικός (azotikos), "no life", because it is asphyxiant.
Though Lavoisier's name was not accepted in English since it was pointed out that all gases but oxygen are either asphyxiant or outright toxic, it is used in many languages (French, Italian, Portuguese, Polish, Russian, Albanian, Turkish, etc.
In earlier times, nitre had been confused with Egyptian "natron" (sodium carbonate) – called νίτρον (nitron) in Greek – which, despite the name, contained no nitrate.
[26] The lack of radial nodes in the 2p subshell is directly responsible for many of the anomalous properties of the first row of the p-block, especially in nitrogen, oxygen, and fluorine.
Thus, despite nitrogen's position at the head of group 15 in the periodic table, its chemistry shows huge differences from that of its heavier congeners phosphorus, arsenic, antimony, and bismuth.
[29] It resembles oxygen with its high electronegativity and concomitant capability for hydrogen bonding and the ability to form coordination complexes by donating its lone pairs of electrons.
This is not possible for its vertical neighbours; thus, the nitrogen oxides, nitrites, nitrates, nitro-, nitroso-, azo-, and diazo-compounds, azides, cyanates, thiocyanates, and imino-derivatives find no echo with phosphorus, arsenic, antimony, or bismuth.
[47] At atmospheric pressure, molecular nitrogen condenses (liquefies) at 77 K (−195.79 °C) and freezes at 63 K (−210.01 °C)[48] into the beta hexagonal close-packed crystal allotropic form.
The less well-characterised ways involve dinitrogen donating electron pairs from the triple bond, either as a bridging ligand to two metal cations (μ, bis-η2) or to just one (η2).
Since N2 is isoelectronic with carbon monoxide (CO) and acetylene (C2H2), the bonding in dinitrogen complexes is closely allied to that in carbonyl compounds, although N2 is a weaker σ-donor and π-acceptor than CO.
The reason for adding gelatin is that it removes metal ions such as Cu2+ that catalyses the destruction of hydrazine by reaction with monochloramine (NH2Cl) to produce ammonium chloride and nitrogen.
Only when heated does it act as a fluorinating agent, and it reacts with copper, arsenic, antimony, and bismuth on contact at high temperatures to give tetrafluorohydrazine (N2F4).
[61] Nitrogen trichloride (NCl3) is a dense, volatile, and explosive liquid whose physical properties are similar to those of carbon tetrachloride, although one difference is that NCl3 is easily hydrolysed by water while CCl4 is not.
Its adduct with ammonia, which was known earlier, is very shock-sensitive: it can be set off by the touch of a feather, shifting air currents, or even alpha particles.
[61][62] For this reason, small amounts of nitrogen triiodide are sometimes synthesised as a demonstration to high school chemistry students or as an act of "chemical magic".
It is rather unreactive (not reacting with the halogens, the alkali metals, or ozone at room temperature, although reactivity increases upon heating) and has the unsymmetrical structure N–N–O (N≡N+O−↔−N=N+=O): above 600 °C it dissociates by breaking the weaker N–O bond.
The latter two compounds are somewhat difficult to study individually because of the equilibrium between them, although sometimes dinitrogen tetroxide can react by heterolytic fission to nitrosonium and nitrate in a medium with high dielectric constant.
Dinitrogen tetroxide is very useful for the preparation of anhydrous metal nitrates and nitrato complexes, and it became the storable oxidiser of choice for many rockets in both the United States and USSR by the late 1950s.
Sodium nitrite is mildly toxic in concentrations above 100 mg/kg, but small amounts are often used to cure meat and as a preservative to avoid bacterial spoilage.
It is also used to synthesise hydroxylamine and to diazotise primary aromatic amines as follows:[70] Nitrite is also a common ligand that can coordinate in five ways.
In concentrated sulfuric acid, nitric acid is protonated to form nitronium, which can act as an electrophile for aromatic nitration:[70] The thermal stabilities of nitrates (involving the trigonal planar NO−3 anion) depends on the basicity of the metal, and so do the products of decomposition (thermolysis), which can vary between the nitrite (for example, sodium), the oxide (potassium and lead), or even the metal itself (silver) depending on their relative stabilities.
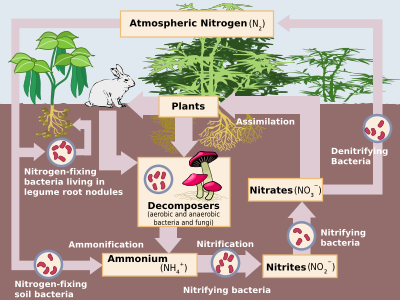
Finally, these organisms die and decompose, undergoing bacterial and environmental oxidation and denitrification, returning free dinitrogen to the atmosphere.
Industrial nitrogen fixation by the Haber process is mostly used as fertiliser, although excess nitrogen–bearing waste, when leached, leads to eutrophication of freshwater and the creation of marine dead zones, as nitrogen-driven bacterial growth depletes water oxygen to the point that all higher organisms die.
[75] In animals, free radical nitric oxide (derived from an amino acid), serves as an important regulatory molecule for circulation.
The gas is mostly used as a low reactivity safe atmosphere wherever the oxygen in the air would pose a fire, explosion, or oxidising hazard.
This may happen with few warning symptoms, since the human carotid body is a relatively poor and slow low-oxygen (hypoxia) sensing system.
[111][112][113] For example, oxygen sensors are sometimes used as a safety precaution when working with liquid nitrogen to alert workers of gas spills into a confined space.









2 converts to colourless dinitrogen tetroxide ( N
2 O
4 ) at low temperatures, and reverts to NO
2 at higher temperatures.