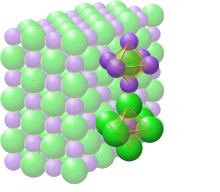
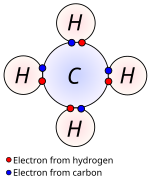
Chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures.
"Constructive quantum mechanical wavefunction interference"[1] stabilizes the paired nuclei (see Theories of chemical bonding).
[8] This is not as a result of reduction in potential energy, because the attraction of the two electrons to the two protons is offset by the electron-electron and proton-proton repulsions.
Instead, the release of energy (and hence stability of the bond) arises from the reduction in kinetic energy due to the electrons being in a more spatially distributed (i.e. longer de Broglie wavelength) orbital compared with each electron being confined closer to its respective nucleus.
Such weak intermolecular bonds give organic molecular substances, such as waxes and oils, their soft bulk character, and their low melting points (in liquids, molecules must cease most structured or oriented contact with each other).
These newly added electrons potentially occupy a lower energy-state (effectively closer to more nuclear charge) than they experience in a different atom.
Specifically, after acknowledging the various popular theories in vogue at the time, of how atoms were reasoned to attach to each other, i.e. "hooked atoms", "glued together by rest", or "stuck together by conspiring motions", Newton states that he would rather infer from their cohesion, that "particles attract one another by some force, which in immediate contact is exceedingly strong, at small distances performs the chemical operations, and reaches not far from the particles with any sensible effect."
Kekulé, A.S. Couper, Alexander Butlerov, and Hermann Kolbe, building on the theory of radicals, developed the theory of valency, originally called "combining power", in which compounds were joined owing to an attraction of positive and negative poles.
In 1904, Nagaoka proposed an alternative planetary model of the atom in which a positively charged center is surrounded by a number of revolving electrons, in the manner of Saturn and its rings.
[12] At the 1911 Solvay Conference, in the discussion of what could regulate energy differences between atoms, Max Planck stated: "The intermediaries could be the electrons.
The Bohr model of the chemical bond took into account the Coulomb repulsion – the electrons in the ring are at the maximum distance from each other.
[15][16] In 1927, the first mathematically complete quantum description of a simple chemical bond, i.e. that produced by one electron in the hydrogen molecular ion, H2+, was derived by the Danish physicist Øyvind Burrau.
[17] This work showed that the quantum approach to chemical bonds could be fundamentally and quantitatively correct, but the mathematical methods used could not be extended to molecules containing more than one electron.
A more practical, albeit less quantitative, approach was put forward in the same year by Walter Heitler and Fritz London.
[18] In 1929, the linear combination of atomic orbitals molecular orbital method (LCAO) approximation was introduced by Sir John Lennard-Jones, who also suggested methods to derive electronic structures of molecules of F2 (fluorine) and O2 (oxygen) molecules, from basic quantum principles.
The equations for bonding electrons in multi-electron atoms could not be solved to mathematical perfection (i.e., analytically), but approximations for them still gave many good qualitative predictions and results.
However this approach has none of the physical pictures of the valence bond and molecular orbital theories and is difficult to extend to larger molecules.
Because atoms and molecules are three-dimensional, it is difficult to use a single method to indicate orbitals and bonds.
In molecular formulas the chemical bonds (binding orbitals) between atoms are indicated in different ways depending on the type of discussion.
Sometimes, even the non-bonding valence shell electrons (with the two-dimensional approximate directions) are marked, e.g. for elemental carbon .
Some chemists may also mark the respective orbitals, e.g. the hypothetical ethene−4 anion (\/C=C/\ −4) indicating the possibility of bond formation.
Ionic bonding is a type of electrostatic interaction between atoms that have a large electronegativity difference.
When such crystals are melted into liquids, the ionic bonds are broken first because they are non-directional and allow the charged species to move freely.
The free movement or delocalization of bonding electrons leads to classical metallic properties such as luster (surface light reflectivity), electrical and thermal conductivity, ductility, and high tensile strength.
They include both Coulombic interactions between partial charges in polar molecules, and Pauli repulsions between closed electrons shells.
[23]: 702 Hydrogen bonds are responsible for the high boiling points of water and ammonia with respect to their heavier analogues.
The properties of the atoms involved can be understood using concepts such as oxidation number, formal charge, and electronegativity.
The concepts of orbital hybridization and resonance augment this basic notion of the electron pair bond.
As approaches for electronic structure theory, both MO and VB methods can give approximations to any desired level of accuracy, at least in principle.
However, at lower levels, the approximations differ, and one approach may be better suited for computations involving a particular system or property than the other.
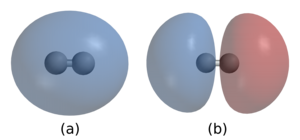
2 . In (a) the two nuclei are surrounded by a cloud of two electrons in the bonding orbital that holds the molecule together. (b) shows hydrogen's antibonding orbital , which is higher in energy and is normally not occupied by any electrons.