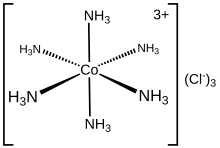
Hexaamminecobalt(III) chloride
The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.
As a manifestation of its inertness, [Co(NH3)6]Cl3 can be recrystallized unchanged from concentrated hydrochloric acid: the NH3 is so tightly bound to the Co(III) centers that it does not dissociate to allow its protonation.
Upon heating, hexamminecobalt(III) begins to lose some of its ammine ligands, eventually producing a stronger oxidant.
The chloride ions in [Co(NH3)6]Cl3 can be exchanged with a variety of other anions such as nitrate, bromide, iodide, sulfamate to afford the corresponding [Co(NH3)6]X3 derivative.
[5] In the biological system, the counterions would more probably be Mg2+, but the heavy atoms of cobalt (or sometimes iridium, as in PDB: 2GIS) provide anomalous scattering to solve the phase problem and produce an electron-density map of the structure.