Cooperative binding
Cooperative binding has been shown to be the mechanism underlying a large range of biochemical and physiological processes.
In 1904, Christian Bohr studied hemoglobin binding to oxygen under different conditions.
In addition, Bohr noticed that increasing CO2 pressure shifted this curve to the right - i.e. higher concentrations of CO2 make it more difficult for hemoglobin to bind oxygen.
at equilibrium as a function of ligand concentration is sigmoidal in shape, as observed by Bohr for hemoglobin, this indicates positive cooperativity.
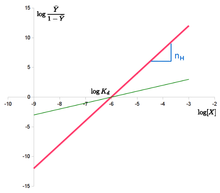
[4] Drawing on observations of oxygen binding to hemoglobin and the idea that cooperativity arose from the aggregation of hemoglobin molecules, each one binding one oxygen molecule, Hill suggested a phenomenological equation that has since been named after him: where
Adair found that the Hill plot for hemoglobin was not a straight line, and hypothesized that binding affinity was not a fixed term, but dependent on ligand saturation.
[5] Having demonstrated that hemoglobin contained four hemes (and therefore binding sites for oxygen), he worked from the assumption that fully saturated hemoglobin is formed in stages, with intermediate forms with one, two, or three bound oxygen molecules.
The formation of each intermediate stage from unbound hemoglobin can be described using an apparent macroscopic association constant
Working on calcium binding proteins, Irving Klotz deconvoluted Adair's association constants by considering stepwise formation of the intermediate stages, and tried to express the cooperative binding in terms of elementary processes governed by mass action law.
[9] By the middle of the 20th century, there was an increased interest in models that would not only describe binding curves phenomenologically, but offer an underlying biochemical mechanism.
The equation below provides the equation for a tetrahedral structure, which would be more accurate in the case of hemoglobin: Based on results showing that the structure of cooperative proteins changed upon binding to their ligand, Daniel Koshland and colleagues[11] refined the biochemical explanation of the mechanism described by Pauling.
[10] The Koshland-Némethy-Filmer (KNF) model assumes that each subunit can exist in one of two conformations: active or inactive.
The Monod-Wyman-Changeux (MWC) model for concerted allosteric transitions[13] went a step further by exploring cooperativity based on thermodynamics and three-dimensional conformations.
It was originally formulated for oligomeric proteins with symmetrically arranged, identical subunits, each of which has one ligand binding site.
According to this framework, two (or more) interconvertible conformational states of an allosteric protein coexist in a thermal equilibrium.
Importantly, all subunits of a molecule change states at the same time, a phenomenon known as "concerted transition".
The allosteric isomerisation constant L describes the equilibrium between both states when no ligand molecule is bound:
The slope of the Hill plot also depends on saturation, with a maximum value at the inflexion point.
[20] The list of molecular assemblies that exhibit cooperative binding of ligands is very large, but some examples are particularly notable for their historical interest, their unusual properties, or their physiological importance.
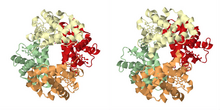
Its quaternary structure, solved by Max Perutz using X-ray diffraction,[21] exhibits a pseudo-symmetrical tetrahedron carrying four binding sites (hemes) for oxygen.
Threonine deaminase was one of the first enzymes suggested to behave like hemoglobin[22] and shown to bind ligands cooperatively.
[25] Although initial models were consistent with four binding sites,[26] its structure was later shown to be hexameric by William Lipscomb and colleagues.
[27] Most ion channels are formed of several identical or pseudo-identical monomers or domains, arranged symmetrically in biological membranes.
[30] Inositol triphosphate (IP3) receptors form another class of ligand-gated ion channels exhibiting cooperative binding.
The molecule does not display a square or tetrahedron structure, but is formed of two lobes, each carrying two EF-hand domains.
A classical example is the binding of the lambda phage repressor to its operators, which occurs cooperatively.
[38] Conversely, examples of negative cooperativity for the binding of transcription factors were also documented, as for the homodimeric repressor of the Pseudomonas putida cytochrome P450cam hydroxylase operon[39] (n=0.56).
In a living cell, ultrasensitive modules are embedded in a bigger network with upstream and downstream components.
The dynamic range limitations imposed by downstream components can produce effective sensitivities much larger than that of the original module when considered in isolation.
This article was adapted from the following source under a CC BY 4.0 license (2013) (reviewer reports): Melanie I Stefan; Nicolas Le Novère (2013).