Covalent organic framework
COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection.
[3] With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties.
[4] This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.
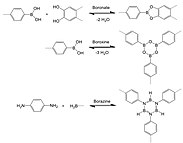
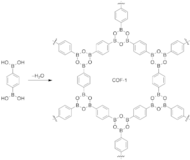
Their crystal structures are entirely held by strong bonds between B, C, and O atoms to form rigid porous architectures with pore sizes ranging from 7 to 27 Angstroms.
[5] The synthesis of 3D COFs has been hindered by longstanding practical and conceptual challenges until it was first achieved in 2007 by Omar M. Yaghi and colleagues, which received the Newcomb Cleveland Prize.
Unlike 0D and 1D systems, which are soluble, the insolubility of 2D and 3D structures precludes the use of stepwise synthesis, making their isolation in crystalline form very difficult.
An almost infinite number of frameworks can be formed through various SBU combinations leading to unique material properties for applications in separations, storage, and heterogeneous catalysis.
[3][12] The bottom-up approach is especially advantageous with respect to materials such as COFs because the synthetic methods are designed to directly result in an extended, highly crosslinked framework that can be tuned with exceptional control at the nanoscale level.
[3] COF materials possess the unique ability for bottom-up reticular synthesis to afford robust, tunable frameworks that synergistically enhance the properties of the precursors, which, in turn, offers many advantages in terms of improved performance in different applications.
As a result, the COF material is highly modular and tuned efficiently by varying the SBUs’ identity, length, and functionality depending on the desired property change on the framework scale.
Through reticular synthesis, it is possible to molecularly engineer modular, framework materials with highly porous scaffolds that exhibit unique electronic, optical, and magnetic properties while simultaneously integrating desired functionality into the COF skeleton.
[18] Since Yaghi and coworkers’ seminal work in 2005, COF synthesis has expanded to include a wide range of organic connectivity such as boron-, nitrogen-, other atom-containing linkages.
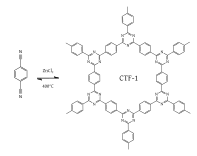
[22] The imine condensation reaction which eliminates water (exemplified by reacting aniline with benzaldehyde using an acid catalyst) can be used as a synthetic route to reach a new class of COFs.
When 1,3,5-triformylphloroglucinol (TFP) is used as one of the SBUs, two complementary tautomerizations occur (an enol to keto and an imine to enamine) which result in a β-ketoenamine moiety[25] as depicted in the DAAQ-TFP[26] framework.
Both DAAQ-TFP and TpOMe-DAQ COFs are stable in acidic aqueous conditions and contain the redox active linker 2,6-diaminoanthroquinone which enables these materials to reversibly store and release electrons within a characteristic potential window.
[31] This high surface area to volume ratio and incredible stability enables the COF structure to serve as exceptional materials for gas storage and separation.
The use of modulators, monofunctional version of precursors, serve to slow the COF formation to allow for more favorable balance between kinetic and thermodynamic control, hereby enabling crystalline growth.
[35] In both previously mentioned COFs, the 2D lattice allows for full π-conjugation in the x and y directions as well as π-conduction along the z axis due to the fully conjugated, aromatic scaffold and π-π stacking, respectively.
[34][35] Emergent electrical conductivity in COF structures is especially important for applications such as catalysis and energy storage where quick and efficient charge transport is required for optimal performance.
For these highly crystalline materials, X-ray diffraction (XRD) is a powerful tool capable of determining COF crystal structure.
[36] Additionally, methods like X-ray photoelectron spectroscopy (XPS), inductively coupled plasma mass spectrometry (ICP-MS), and combustion analysis can be used to identify elemental composition and ratios.
[42] Most studies to date have focused on the development of synthetic methodologies with the aim of maximizing pore size and surface area for gas storage.
[44] MOF under solvent-free conditions can also be used for catalytic activity in the cycloaddition of CO2 and epoxides into cyclic organic carbonates with enhanced catalyst recyclability.
[46] Due to the ability to introduce diverse functionality into COFs’ structure, catalytic sites can be fine-tuned in conjunction with other advantageous properties like conductivity and stability to afford efficient and selective catalysts.