Diphenylketene
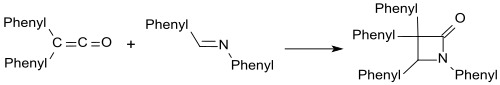
The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.
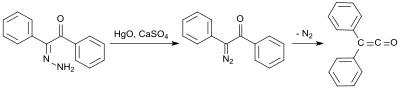
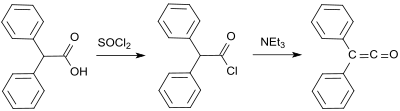
[3] The first synthesis by H. Staudinger was based on 2-chlorodiphenylacetyl chloride (prepared from benzilic acid and thionyl chloride[4]) from which two chlorine atoms are cleaved with zinc in a dehalogenation reaction:[2] An early synthesis uses benzilmonohydrazone (from Diphenylethanedione and hydrazine hydrate[5]), which is oxidized with mercury(II)oxide and calcium sulfate to form mono-diazoketone, and is then converted into the diphenylketene at 100 °C under nitrogen elimination in 58% yield:[6] A further early diphenylketene synthesis originates from Eduard Wedekind, who had already obtained diphenyl ketene in 1901 by the dehydrohalogenation of diphenylacetyl chloride with triethylamine, without isolation and characterization though.
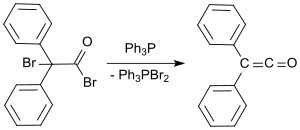
[10] Recently, a synthesis of diphenyl ketene from diphenylacetic acid and the Hendrickson reagent (triphenylphosphonium anhydride-trifluoromethanesulfonate)[11] with water elimination in 72% yield has been reported.
[12] Diphenyl ketene is at room temperature an orange-colored to red oil (with the color of concentrated potassium dichromate solution[2]) which is miscible with nonpolar organic solvents (such as diethyl ether, acetone, benzene, tetrahydrofuran, chloroform)[13] and solidifies in the cold forming yellow crystals.
[2] The compound is easily oxidized by air but can be stored in tightly closed containers at 0 °C for several weeks without decomposition[9] or in a nitrogen atmosphere with the addition of a small amount of hydroquinone as a polymerization inhibitor.
[6] Diphenylketene can undergo attack from a host of nucleophiles, including alcohols, amines, and enolates with fairly slow rates.