Discovery and development of beta2 agonists
The aim of the drug development through the years has been to minimise side effects, achieve selectivity and longer duration of action.
Albuterol and terbutaline gave fewer side effects, such as increased heart rate, than isoproterenol.
This long duration of action made the treatment for severe asthma and COPD more convenient for the patients because it is inhaled twice a day.
Its duration of action lasts for 24 hours which should improve patients' compliance and make the treatment more convenient.
When this constriction occurs, the airways get narrow and it causes symptoms like wheezing, chest tightness, shortness of breath, and coughing.
Smoking is the main risk factor but inhalation of toxic and harmful particles and gases can also cause the disease.
[10] The kinetics of airway smooth muscle relaxation, as long as the onset and duration of bronchodilation in asthmatic patients, are reflected by the difference in the mechanism of interaction of short- (SABAs) and long-acting β2-agonists (LABAs) and the β2-receptor.
[12] Sulfate conjugates are the main metabolites; protein binding is rather weak and only insignificant interactions have been found with other drugs.
Also, the long duration of action for salmeterol is related to increased lipophilicity of the molecules, allowing it to remain for a longer time in the lungs.
[15] G-protein coupled receptors consist of single polypeptide chains of 300-600 amino acids and span the plasma membrane seven times.
[16] When the β2-agonist binds and activates the β2-adrenoreceptor intracellular signaling becomes largely affected through cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA).
[11][16] The mechanism by which cAMP induces relaxation in airway smooth muscle cells is not fully understood.
It is believed that cAMP catalyzes the activation of PKA, which in turn phosphorylates key regulatory proteins involved in the control of muscle tone.
Hydrogen bonds are formed from the hydroxyl groups, linked to the catechol ring, to Ser-204 and Ser-207 in helix 5.
The beta-carbon is chiral and must have the R-configuration so that the beta-hydroxyl group is oriented towards the Asn-293 residue in helix 6 to form a hydrogen bond essential to binding to the beta-2 receptor.
et al. introduced the plasmalemma diffusion microkinetic theory, explaining what happens to the β2- agonist in the cell membrane lipid bilayer and in the aqueous biophase closest to the binding site of the β2-adrenoceptor.
It is postulated that the plasmalemma lipid bilayer of airway smooth muscles acts as a depot for β2-adrenoceptor agonists.
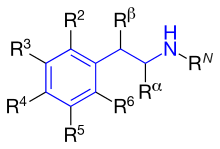
[17] The fundamental pharmacophore for all adrenergic agonists is a substituted phenethylamine which increases the duration of action.
[14] Adrenergic agonists that are selective for the β2 subtype cause bronchial dilation and might be expected to relieve the bronchospasm of an asthmatic attack.
One approach to avoid these side effects is to use structurally different features that may minimize absorption into systemic circulation.
Direct-acting analog binds the β-adrenergic receptors directly and generates sympathetic response.
However, an ethyl group seems to cause increased adverse effects and low β2-receptor potency compared to other β2-selective agonists.
As noted earlier, all marketed β2-agonists have a hydroxyl group in this position which makes the compound chiral, and is active when it has the (R)-configuration.
[14] R5 or R3: Hydroxyl group placed on carbon number 5 or 3 (meta position) gives direct action to the β-adrenergic receptor.
[14] Summarizing a few β2-adrenoceptor agonists and their structure activity shows how they act differently referred to potency, selectivity, affinity and duration of action (see table 1): The β2-agonsist that are clinically used are all substituted β-phenethylamine (see figure 5) and they have three kinds of phenyl rings shown in figure 4.