Discovery and development of gastrointestinal lipase inhibitors
Their mode of action is to inhibit gastric and pancreatic lipases, enzymes that play an important role in the digestion of dietary fat.
[6] Pancreatic lipase inhibitor was originally discovered and isolated from fermented broth of the Streptomyces toxytricini bacterium in 1981 and named lipstatin.
Most reported side effects are gastrointestinal; including liquid stools, steatorrhea and abdominal pain.
These inhibitors bind covalently as an ester to the serine hydroxyl group at the active site on pancreatic- and gastric lipases and form a stable complex.
The inactive lipase is incapable of hydrolysing fats into absorbable fatty acids and monoglycerides, therefore triglycerides are excreted undigested with faeces.
[citation needed] With this mode of action calorie uptake from fat in food is limited, hence body weight is reduced.
More than 95% of fat in food consists of triglycerides, which are categorized based on the length of fatty acids connected to glyceride backbone.
[19] For that reason lipases in the gastrointestinal tract must hydrolyse it to smaller molecules, free fatty acids and monoglyceride,[20] before absorption can occur.
[20] Fat digestion begins when gastric lipase hydrolyses dietary triglycerides, by cleaving only one long-, medium- or short-acyl chain from the glyceride backbone and release free fatty acids and diacylglycerols.
[21] The hydrophobic region around Ser152, which has the hexapeptide sequence Val-Gly-His-Ser-Gln-Gly, is essential for the catalytic activity of gastric lipase.
[21] Pancreatic lipase is the most important lipolytic enzyme in the gastrointestinal tract[21] and is essential for fat digestion.
[20] Pancreatic lipase hydrolyses triglycerides and diglycerides by cleaving acyl chains at the sn-1 and sn-3 position[21] and releases free fatty acids and 2-monoglycerides.
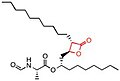
The most important and necessary chemical group for the binding and activity of these compounds is the β-lactone (beta-lactone) ring which is the central pharmacophore.
[5][8] The lactone ring structure binds irreversibly to the active site of the lipase and forms covalent bond, which leads to inhibition.
[1] Besides the role of the β-lactone ring in structure-activity relationship, the nature of the functional groups (e.g. ester or ether and the chain length at the β-site) also matter.
Those who have a β-lactone ring are lipstatin, valilactone, percyquinin, panclicin A-E, ebelactone A and B, vibralactone and esterastin.
[35] Orlistat inhibits FAS with the same mechanism as it does with pancreatic lipase, that is by binding covalently to the active serine site.
[35] This effect of orlistat as a FAS-inhibitor was first identified in a high throughput screening for enzymes with prostate cancer inhibition activity.
[34][37] Orlistat works locally in the intestines as a lipase inhibitor, and therefore suffers from several limitations in its development as a systemic drug.