Cereblon E3 ligase modulator
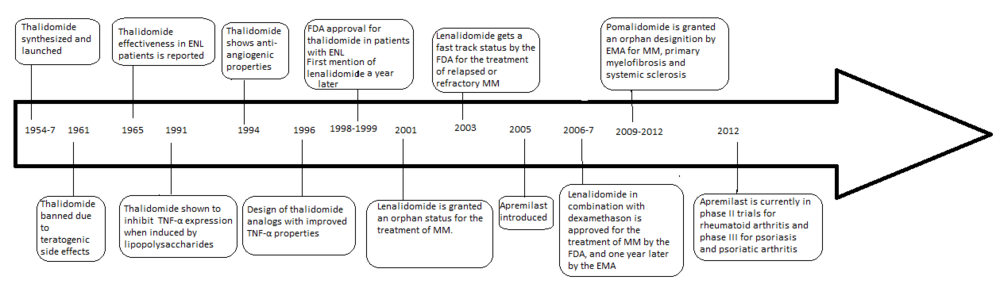
The development of analogs of thalidomide was precipitated by the discovery of the anti-angiogenic and anti-inflammatory properties of the drug yielding a new way of fighting cancer as well as some inflammatory diseases after it had been banned in 1961.
In 1998 thalidomide was approved by the U.S. Food and Drug Administration (FDA) for use in newly diagnosed multiple myeloma (MM) under strict regulations.
[2] This has led to the development of a number of analogs with fewer side effects and increased potency which include lenalidomide and pomalidomide, which are currently marketed and manufactured by Celgene.
The problems with thalidomide were, aside from the teratogenic side effects, both high incidence of other adverse reactions along with poor solubility in water and absorption from the intestines.
[3][4] Adverse reactions include peripheral neuropathy in large majority of patients, constipation, thromboembolism along with dermatological complications.
Thalidomide was discovered to inhibit tumour necrosis factor-alpha (TNF-α) in 1991 (5a Sampaio, Sarno, Galilly Cohn and Kaplan, JEM 173 (3) 699–703, 1991) .
The discovery of the anti-inflammatory, anti-angiogenic and anti-tumor activities of thalidomide increased the interest of further research and synthesis of safer analogs.
[8] The primary use of IMiDs in medicine is in the treatment of cancers and autoimmune diseases (including one that is a response to the infection leprosy).
Orphan indications by the FDA include graft-versus-host disease, mycobacterial infection, recurrent aphthous ulcers, severe recurrent aphthous stomatitis, primary brain malignancies, AIDS-associated wasting syndrome, Crohn's disease, Kaposi's sarcoma, myelodysplastic syndrome and hematopoietic stem cell transplantation.
[21][22] Lenalidomide is approved in nearly 70 countries, in combination with dexamethasone for the treatment of patients with MM who have received at least one prior therapy.
Lenalidomide is also approved for transfusion-dependent anemia due to low or intermediate-1-risk myelodysplastic syndromes associated with a deletion 5q cytogenetic abnormality with or without additional cytogenetic abnormalities in the U.S., Canada, Switzerland, Australia, New Zealand, Malaysia, Israel and several Latin American countries, while marketing authorization application is currently being evaluated in a number of other countries.
Cereblon is a 51 kDa protein localized in the cytoplasm, nucleus and peripheral membrane of cells in numerous parts of the body.
This disrupts the positive feedback loop between the two growth factors, possibly causing both multiple birth defects and anti-myeloma effects.
Findings also support the hypothesis that an increase in the expression of cereblon is an essential element of the anti-myeloma effect of both lenalidomide and pomalidomide.
[34] Thalidomide and its analogs, lenalidomide and pomalidomide, are believed to act in a similar fashion even though their exact mechanism of action is not yet fully understood.
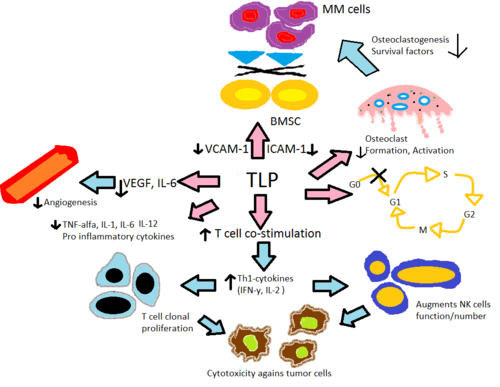
[35] The mechanism is believed to be through enhanced degradation of TNF-α mRNA, resulting in diminished amounts of this pro-inflammatory cytokine secreted.
[36] This explains the effect of thalidomide when given to ENL patients, as they commonly have high levels of TNF-α in their blood and in dermatological lesions.
[8] In contrast, in vitro assay demonstrated that TNF-α is actually enhanced in T-cell activation, where CD4+ and CD8+ T lymphocytes were stimulated by anti-CD3[8][35] which was later confirmed in an early phase trials involving solid tumors and inflammatory dermatologic diseases.
In addition, in vitro data suggests pomalidomide reverts Th2 cells into Th1 by enhancing transcription factor T-bet.
[36] Thalidomide and its analogs directly decrease the up-regulation of IL-6 and indirectly through TNF-α, thereby reducing the secretion of adhesion molecules leading to fewer MM cells adhering to BMSC.
Other additions of longer and bigger groups at the C4 and C5 position of the phthaloyl ring system of thalidomide, some with an olefin functionality, have been tested with various results.
Increased inhibitory effect, compared to thalidomide, was noticed with the groups that had an oxygen atom attached directly to the C5 or C4 olefin.
[16] When the substitution at the 4 (Z) location on the phthaloyl ring was examined, hydroxyl and methoxy groups seem to make the analog a less potent PDE4 inhibitor.
The downside of this process however is that the last step requires a high-temperature melt reaction which demands multiple recrystallizations and is not compliant with standard equipment.
This route uses L-glutamine rather than L-glutamic acid as a starting material and by letting it react with N-carbethoxyphthalimide gives N-phthaloyl-L-glutamine (4), with 50–70% yield.
The substance 4 is then stirred in a mixture with carbonyldiimidazole (CDI) with enough 4-dimethylaminopyridine (DMAP) in tetrahydrofuran (THF) to catalyze the reaction and heated to reflux for 15–18 hours.
[46] Both of the amino analogs are prepared from the condensation of 3-aminopiperidine-2,6-dione hydrochloride (Compound 3) which is synthesized in a two step reaction from commercially available Cbz-L-glutamine.
To remove the Cbz protecting group hydrogenolysis, under 50–60 psi of hydrogen with 10% Pd/C mixed with ethyl acetate and HCl, was performed.
The formulated hydrochloride (Compound 3 in Scheme 3) was then reacted with 3-nitrophthalic anhydride in refluxing acetic acid to yield the 4-nitro substituted thalidomide analog and the nitro group then reduced with hydrogenation to give pomalidomide.
[44] Lenalidomide is synthesized in a similar way using compound 3 (3-aminopiperidine-2,6-dione) treated with a nitro-substituted methyl 2-(bromomethyl) benzoate, and hydrogenation of the nitro group.