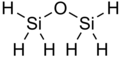
Disiloxane
The structure of disiloxane has been studied by a variety of spectroscopic methods such as electron diffraction,[1] X-ray crystallography,[2] dipole moment, and nuclear magnetic resonance spectroscopy.
[5] In addition to studies of bond angles, vibrational analyses have also been done to determine the symmetry elements of disiloxane.
[citation needed] While disiloxane itself has a bent molecular geometry at oxygen, the related compound hexaphenyldisiloxane, Ph3Si−O−SiPh3, has an Si−O−Si angle of 180°.
[6] Synthesis of disiloxane is typically done by taking a hydrosilane species with a substituent leaving group and reacting it with water to produce silanol.
The properties that disiloxane exhibits in these products include fast drying, oil reducing, moisturizing, skin conditioning, and defoaming agent (preventing formation of foam).
Siloxanes of many kinds are found to be extremely safe for topical use but can be dangerous if ingested in large quantities.