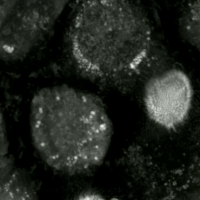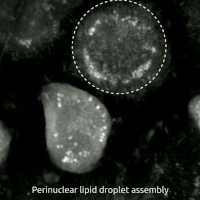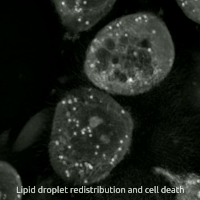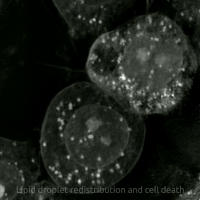
Ferroptosis
Ferroptosis (also known as oxytosis) is a type of programmed cell death dependent on iron and characterized by the accumulation of lipid peroxides.
Ferroptosis is biochemically, genetically, and morphologically distinct from other forms of regulated cell death such as apoptosis and necroptosis.
[1] Oxytosis/ferroptosis can be initiated by the failure of the glutathione-dependent antioxidant defenses, resulting in unchecked lipid peroxidation and eventual cell death.
[5] In 1989, work by the groups of Joseph T. Coyle and Ronald Schnaar showed in a neuronal cell line that excess exposure to glutamate or lowered cystine causes a decrease in glutathione levels, an accumulation in intracellular peroxides, and cytotoxicity.
[7][8] Later work by Pamela Maher and David Schubert noted the distinction of this cell death process from apoptosis, describing it as oxidative glutamate toxicity or oxytosis.
[9][10][11] In 2012, a study by Brent Stockwell and Scott Dixon characterized the iron dependence of this cell death process and coined the term ferroptosis.
[35][36] FSP1 enzymatically reduces non-mitochondrial coenzyme Q10 (CoQ10), thereby generating a potent lipophilic antioxidant that suppresses the propagation of lipid peroxides.
[37] A similar mechanism for a cofactor moonlighting as a diffusable antioxidant was discovered in the same year for tetrahydrobiopterin (BH4), a product of the rate-limiting enzyme GTP cyclohdrolase 1 (GCH1).
[citation needed] Ferroptosis was initially characterized in human cell lines and has been since found to occur in other mammals (mice),[42] avians (chicken),[43] worms (C. elegans),[44][45] and plants (A. thaliana,[46][47] T. aestivum L.,[48] and others).
[43] The exact pro-ferroptotic signal that is transmitted between cells and the manner by which these ferroptotic waves are bounded remain to be characterized.
[67][68][69] A pair of studies in 2017 found that these cancer cells in this therapy-induced drug-resistant state exhibit a greater dependence on GPX4 to suppress ferroptosis.
[citation needed] Recent studies have suggested that oxytosis/ferroptosis contributes to neuronal cell death after traumatic brain injury.
[82][83][84] Reagents to image ferroptosis have been developed to monitor anticancer drug-induced acute kidney injury in mouse models.
[89][90][91] Neutrophil ferroptosis is prevalent in patients with SLE and is induced by autoantibodies and interferon-alpha (IFN-α), which suppress GPX4 expression via the transcriptional repressor CREMα.
[93] Subsequent study found that small intestinal epithelial cells (IECs) from Crohn's disease patient samples show reduced GPX4 expression and activity and lipid peroxidation.
[94] The same study found that dietary lipids in Western diets such as the PUFA arachidonic acid can trigger enteritis resembling Crohn's disease in a mouse model.
Specifically, in cellular and lysate contexts, ML210 undergoes ring-opening hydrolysis followed by a retro-Claisen-like condensation and ring-closing hydration to yield an unstable furoxan.
Work from Kojin Therapeutics and Ono Pharmaceutical has demonstrated that inhibition of glutamate-cysteine ligase (GCL), the rate-limiting enzyme in GSH biosynthesis, induces ferroptosis in cancer cell lines.