Gaseous signaling molecules
[1][2] The levels of O2 in cells or organisms must be tighly regulated to ensure normoxic and not uncontrolled hypoxic or anoxic or hyperoxic states.
Mammalian cells have a specialized gasoreceptor soluble guanylyl cyclase that bind to NO and trigger NO-dependent cellular signaling.
[6] Some authors have shown that this process of NO reduction to N2O takes place in hepatocytes, specifically in their cytoplasm and mitochondria, and suggested that the N2O can possibly be produced in mammalian cells.
[8] In 1981, it was first suggested from clinical work with nitrous oxide (N2O) that a gas had a direct action at pharmacological receptors and thereby acted as a neurotransmitter.
[20] The ability of nitrous oxide to alter the structure and function of these proteins was demonstrated by shifts in infrared spectra of cysteine thiols of hemoglobin[21] and by partial and reversible inhibition of cytochrome oxidase.
[26][27][28] Other than that, some authors think also that those macrocyclic compounds of carbon suboxide can possibly diminish free radical formation and oxidative stress and play a role in endogenous anticancer protective mechanisms, for example in the retina.
[30] Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors and abolishes the Hering–Breuer inflation reflex.
Endogenous sulfur dioxide lowered lipid peroxidation, free radical formation, oxidative stress and inflammation during an experimental lung damage.
Conversely, a successful lung damage caused a significant lowering of endogenous sulfur dioxide production, and an increase in lipid peroxidation, free radical formation, oxidative stress and inflammation.
Conversely, adding acetylcysteine or glutathione to the rat diet increased the amount of endogenous SO2 produced and decreased the lung damage, the free radical formation, oxidative stress, inflammation and apoptosis.
[31] Endogenous sulfur dioxide may play a role in regulating cardiac and blood vessel function, and aberrant or deficient sulfur dioxide metabolism can contribute to several different cardiovascular diseases, such as arterial hypertension, atherosclerosis, pulmonary arterial hypertension, stenocardia.
Authors considered homocysteine to be one of useful biochemical markers of disease severity and sulfur dioxide metabolism to be one of potential therapeutic targets in those patients.
Hyperammonemia contributes to the confusion and coma of hepatic encephalopathy, as well as the neurologic disease common in people with urea cycle defects and organic acidurias.
After formation of ammonium from glutamine, α-ketoglutarate may be degraded to produce two molecules of bicarbonate, which are then available as buffers for dietary acids.
[46] It acts at trace levels throughout the life of the plant by stimulating or regulating the ripening of fruit, the opening of flowers, and the abscission (or shedding) of leaves.
Commercial ripening rooms use "catalytic generators" to make ethylene gas from a liquid supply of ethanol.
In 1864, it was discovered that gas leaks from street lights led to stunting of growth, twisting of plants, and abnormal thickening of stems.
[51] Ethylene is produced from essentially all parts of higher plants, including leaves, stems, roots, flowers, fruits, tubers, and seeds.
During the life of the plant, ethylene production is induced during certain stages of growth such as germination, ripening of fruits, abscission of leaves, and senescence of flowers.
Ethylene production can also be induced by a variety of external aspects such as mechanical wounding, environmental stresses, and certain chemicals including auxin and other regulators.
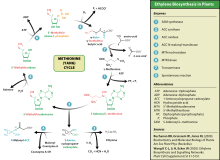
[52] Ethylene is biosynthesized from the amino acid methionine to S-adenosyl-L-methionine (SAM, also called Adomet) by the enzyme Met Adenosyltransferase.
ACC synthesis increases with high levels of auxins, especially indole acetic acid (IAA) and cytokinins.
[46] Environmental cues such as flooding, drought, chilling, wounding, and pathogen attack can induce ethylene formation in plants.
[71] Endogenous ethylene oxide, just as like environmental (exogenous) one, can alkylate guanine in DNA, forming an adduct 7-(2-hydroxyethyl)-guanine, and this poses an intrinsic carcinogenic risk.