Glucagon
It raises the concentration of glucose and fatty acids in the bloodstream and is considered to be the main catabolic hormone of the body.
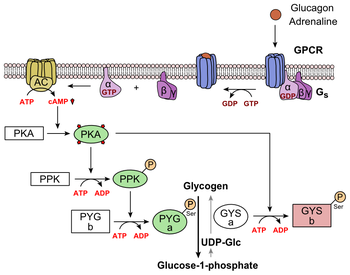
Glucagon causes the liver to engage in glycogenolysis: converting stored glycogen into glucose, which is released into the bloodstream.
The hormone is synthesized and secreted from alpha cells (α-cells) of the islets of Langerhans, which are located in the endocrine portion of the pancreas.
[6] Proglucagon is then cleaved by proprotein convertase 2 to glucagon (amino acids 33-61) in pancreatic islet α cells.
[28] Malonyl-CoA is a product formed by ACC during denovo synthesis and an allosteric inhibitor of Carnitine palmitoyltransferase I (CPT1), a mitochondrial enzyme important for bringing fatty acids into the intermembrane space of the mitochondria for β-oxidation.
[29] Glucagon decreases malonyl-CoA through inhibition of acetyl-CoA carboxylase and through reduced glycolysis through its aforementioned reduction in Fructose 2,6-bisphosphate.
Abnormally elevated levels of glucagon may be caused by pancreatic tumors, such as glucagonoma, symptoms of which include necrolytic migratory erythema,[30] reduced amino acids, and hyperglycemia.
[31] Elevated glucagon is the main contributor to hyperglycemic ketoacidosis in undiagnosed or poorly treated type 1 diabetes.
As the beta cells cease to function, insulin and pancreatic GABA are no longer present to suppress the freerunning output of glucagon.
As a result, glucagon is released from the alpha cells at a maximum, causing a rapid breakdown of glycogen to glucose and fast ketogenesis .
[32] It was found that a subset of adults with type 1 diabetes took 4 times longer on average to approach ketoacidosis when given somatostatin (inhibits glucagon production) with no insulin.
[citation needed] The absence of alpha cells (and hence glucagon) is thought to be one of the main influences in the extreme volatility of blood glucose in the setting of a total pancreatectomy.
[6] In 1922, C. Kimball and John R. Murlin identified a component of pancreatic extracts responsible for this blood sugar increase, terming it "glucagon", a portmanteau of "glucose agonist".
[6][33] In the 1950s, scientists at Eli Lilly isolated pure glucagon, crystallized it, and determined its amino acid sequence.
[36] In 1979, while working in Joel Habener's laboratory at Massachusetts General Hospital, Richard Goodman collected islet cells from Brockman bodies of American anglerfish in order to investigate somatostatin.
[37] By splicing DNA from anglerfish islet cells into bacteria, Goodman was able to identify the gene which codes for somatostatin.