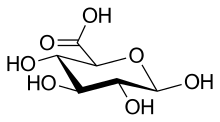
Glucuronic acid
Glucuronic acid, like its precursor glucose, can exist as a linear (carboxo-)aldohexose (<1%), or as a cyclic hemiacetal (furanose or pyranose).
Due to ring closure, cyclic sugars have another asymmetric carbon atom (C-1), resulting in two more stereoisomers, named anomers.
A laboratory synthesis of a uronic acid from an aldose requires protecting the aldehyde and hydroxy groups from oxidation, for example by conversion to cyclic acetals (e. g., acetonides).
Glucuronidation occurs mainly in the liver, although the enzymes responsible for its catalysis, UDP-glucuronyltransferases (UDP-GT), have been found in all major body organs, e.g., intestine, kidneys, brain, adrenal gland, spleen, and thymus.
Neonates are deficient in this conjugating system, making them particularly vulnerable to drugs such as chloramphenicol, which is inactivated by the addition of glucuronic acid, resulting in gray baby syndrome.
It is possible to exhaust the body's supply of glucuronic acid by combining multiple drugs/substances whose metabolism and excretion are primarily or entirely dependent on glucuronidation.
In the most severe cases, permanent and debilitating organ damage (particularly the liver, kidneys, heart, and brain), and even death, have been known to occur.
Ethanol, morphine, paracetamol (acetaminophen), cyclooxygenase inhibitors (NSAIDs), endogenous steroids, and certain benzodiazepines are all capable of contributing to GCA depletion, with ethanol and acetaminophen being the most commonly implicated substances involved in cases of accidental overdoses which have been positively attributed to glucuronic acid depletion.
Increased GCA activity results in a decrease of the concentration and metabolic half-life of glucuronic acid substrates, causing the plasma levels of glucuronidated drugs to fall below their therapeutic threshold.
A select number of antidepressants and a wide range of anti-psychotic agents are glucuronidation ligands, but due to their delayed mechanism of action and pharmacokinetic properties the decrease of their plasma concentrations may not be immediately apparent and tends to present as a sudden and intense relapse of symptoms instead of a gradual regression to the behaviors and thought patterns exhibited by the patient prior to the initiation of their pharmacological treatment.
Covalent binding of the aglycone portions of several carboxylic acid (ester) glucuronides is known to occur to nucleophilic sites on serum albumin via transacylation reactions, for example.
[5] Glucuronic acid, as well as the glucuronidated metabolite of ethanol, ethyl glucuronide (ETG), act on toll-like receptor 4 to aggravate both acute and chronic inflammatory conditions as well as increasing the perceived severity of pain in patients with chronic pain conditions, via up-regulation of the production and release of endogenous inflammatory signaling molecules within the body.
E. coli produces the enzyme β-glucuronidase, which hydrolyzes the MUG molecule to a fluorescent product that is detectable under ultraviolet light.