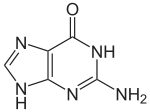
Guanine
With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with conjugated double bonds.
In cytosine, the amino group acts as the hydrogen bond donor and the C-2 carbonyl and the N-3 amine as the hydrogen-bond acceptors.
Its high melting point of 350 °C reflects the intermolecular hydrogen bonding between the oxo and amino groups in the molecules in the crystal.
Because of this intermolecular bonding, guanine is relatively insoluble in water, but it is soluble in dilute acids and bases.
In 1984, Yuasa reported a 0.00017% yield of guanine after the electrical discharge of NH3, CH4, C2H6, and 50 mL of water, followed by a subsequent acid hydrolysis.
As the Oxford English Dictionary notes, guanine is "A white amorphous substance obtained abundantly from guano, forming a constituent of the excrement of birds".
[12] Spiders, scorpions, and some amphibians convert ammonia, as a product of protein metabolism in the cells, to guanine, as it can be excreted with minimal water loss.
[13] On 8 August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting building blocks of DNA and RNA (guanine, adenine and related organic molecules) may have been formed extra-terrestrially in outer space.