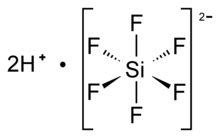
Hexafluorosilicic acid
Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion.
The resulting hexafluorosilicic acid is almost exclusively consumed as a precursor to aluminum trifluoride and synthetic cryolite, which are used in aluminium processing.
[5] As a by-product, approximately 50 kg of hexafluorosilicic acid is produced per tonne of HF owing to reactions involving silica-containing mineral impurities.
Thus, for example, hexafluorosilicic acid pure or in oleum solution evolves silicon tetrafluoride until the residual hydrogen fluoride re-establishes equilibrium:[7] In alkaline-to-neutral aqueous solutions, hexafluorosilicic acid readily hydrolyzes to fluoride anions and amorphous, hydrated silica ("SiO2").
[7] At the concentrations usually used for water fluoridation, 99% hydrolysis occurs:[6][8] Neutralization of solutions of hexafluorosilicic acid with alkali metal bases produces the corresponding alkali metal fluorosilicate salts: The resulting salt Na2SiF6 is mainly used in water fluoridation.
At room temperature 15-30% concentrated hexafluorosilicic acid undergoes similar reactions with chlorides, hydroxides, and carbonates of alkali and alkaline earth metals.
Some rare minerals, encountered either within volcanic or coal-fire fumaroles, are salts of the hexafluorosilicic acid.
Examples include ammonium hexafluorosilicate that naturally occurs as two polymorphs: cryptohalite and bararite.