Hindered amine light stabilizers
[2][3] They are also increasingly being used as thermal stabilizers,[4][5] particularly for low and moderate level of heat, however during the high temperature processing of polymers (e.g. injection moulding) they remain less effective than traditional phenolic antioxidants.
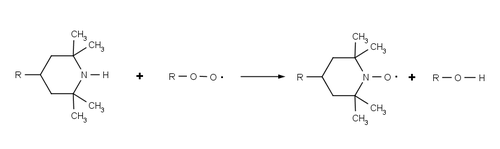
The use of a hindered amine possessing no alpha-hydrogens prevents the HALS being converted into a nitrone species and piperidines are resistant to intramolecular Cope reactions.
[9] In commercial HALS the reactive piperidine group is usually bonded to bulky chemical scaffold, in order to reduce its volatility during the melt processing of plastic.
Even though HALS are extremely effective in polyolefins, polyethylene and polyurethane, they are ineffective in polyvinyl chloride (PVC).
It is thought that their ability to form nitroxyl radicals is disrupted due them being readily protonated by HCl released by dehydrohalogenation of PVC.