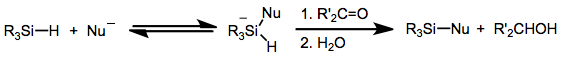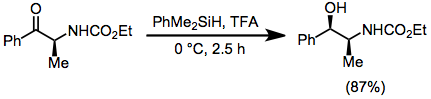Hydrosilanes
One example is triethoxysilane: Organohydrosilanes can be prepared by partial hydrosilation of silane itself: In the laboratory, hydrosilanes classically are prepared by treating chlorosilanes with hydride reagents, such as lithium aluminium hydride: The silicon-to-hydrogen bond is longer than the C–H bond (148 compared to 105 pm).
Generally silyl hydrides are colourless with physical properties (solubility, volatility) comparable to hydrocarbons.
Setting aside silane itself, for which is used mainly in the microelectronics industry as a source of Si, hydrosilanes participate in many reactions.
[2] In the presence of fluoride ions, hydrosilanes reversibly form hypervalent fluorosilicates with the formula R3Si(F)H−).
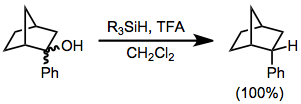
In this type of reaction, carbocations are generated by the action of strong Lewis or Brønsted acids in the presence of hydrosilanes, which then transfer hydride.
[6] Such adducts represent models for and competitors with the oxidative addition of the Si-H bond.