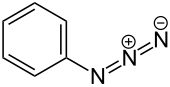
Phenyl azide
The structure consists of a linear azide substituent bound to a phenyl group.
[2][3] Phenyl azide is prepared by the diazotization of phenylhydrazine with nitrous acid:[4] Aryl iodides bearing electron-withdrawing substituents undergo metathesis with sodium azide in the presence of Cu(I), sodium ascorbate, and N,N'-dimethylethane-1,2-diamine (DMEDA):[5] It can also be prepared by condensation of benzenediazonium salt with toluenesulfonamide, followed by hydrolysis.
Phenyl azide cycloadds to alkenes and especially alkynes, particularly those bearing electronegative substituents.
In a classic example of click chemistry, phenyl azide and phenylacetylene react to give diphenyl triazole.
Organic Syntheses recommends a vacuum of 5mm Hg to give a boiling point of "66–68 °C/21 mm.