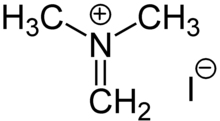
Iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure [R1R2C=NR3R4]+.
Iminium cations adopt alkene-like geometries: the central C=N unit is nearly coplanar with all four substituents.
[3][4] Iminium cations are obtained by protonation and alkylation of imines: They also are generated by the condensation of secondary amines with ketones or aldehydes: This rapid, reversible reaction is one step in "iminium catalysis".
Pyridoxal phosphate reacts with amino acids to give iminium derivatives.
The isomerization is catalyzed by nucleophiles, which add to the unsaturated carbon, breaking the C=N double bond.