Integral membrane protein
Such proteins can only be separated from the membranes by using detergents, nonpolar solvents, or sometimes denaturing agents.
Three-dimensional structures of ~160 different integral membrane proteins have been determined at atomic resolution by X-ray crystallography or nuclear magnetic resonance spectroscopy.
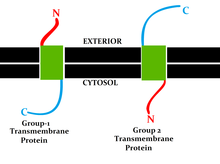
Search integral membrane proteins in the PDB (based on gene ontology classification) IMPs can be divided into two groups:[citation needed] The most common type of IMP is the transmembrane protein, which spans the entire biological membrane.
[4] Integral monotopic proteins are permanently attached to the cell membrane from one side.
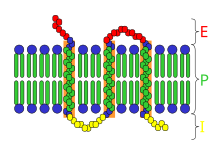
[8] As an example of the relationship between the IMP (in this case the bacterial phototrapping pigment, bacteriorhodopsin) and the membrane formed by the phospholipid bilayer is illustrated below.
In this case the integral membrane protein spans the phospholipid bilayer seven times.