Kuwajima Taxol total synthesis
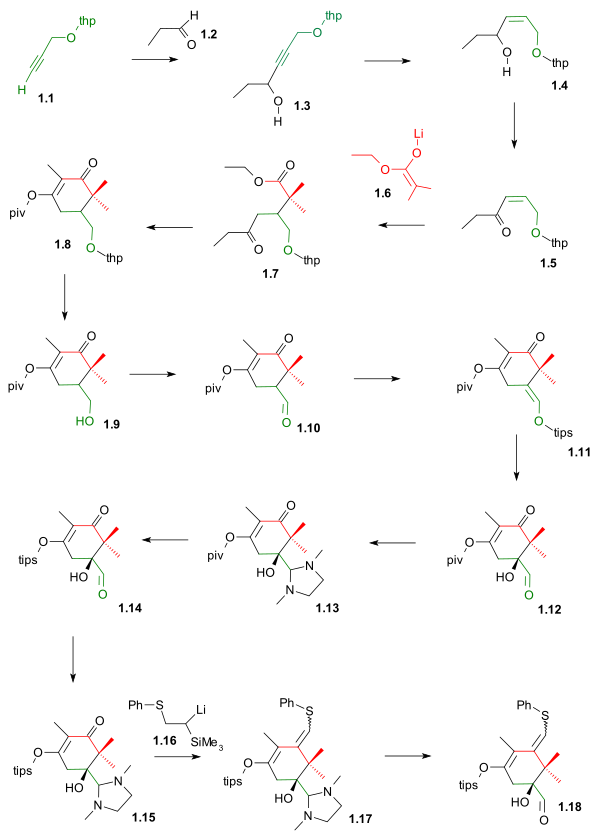
The TIPS silyl enol ether 1.11 was formed by reaction with the triflate TIPSOtf and DBU in DMAP setting the stage for asymmetric dihydroxylation to hydroxyaldehyde 1.12.
The C10 fragment was then introduced by the lithium salt of Trimethyl(phenylthiomethyl)silane 1.16 in a Peterson olefination to the sulfide 1.17 followed by deprotection to completed ring A 1.18.
The diol in 2.3 was protected as the boronic ester 2.4 preparing the molecule for upper part ring closure with tin tetrachloride to tricycle 2.5 in a Grob fragmentation-like reaction.
The enol was protected as the TBS ether 3.10 allowing for the reduction of the nitrile group first to the aldehyde with DIBAL and then on to the alcohol 3.11 with Lithium aluminium hydride.
These preambles facilitated the introduction of the final missing C20 fragment as the Grignard reagent trimethylsilylmethylmagnesium bromide which coupled with the triflate in a tetrakis(triphenylphosphine)palladium(0) catalysed reaction to the silane 4.8.
Next dihydroxylation with Osmium(VIII) oxide formed the diol 4.13 with the primary alcohol on addition of base DBU displacing the chlorine atom in a nucleophilic aliphatic substitution to oxetane 4.14.
The C13 alcohol protective group was removed in 5.10 (TASF) enabling the tail addition of Ojima lactam 5.11 (this step is common with all total synthetic efforts to date) to 5.12 with Lithium bis(trimethylsilyl)amide.