Danishefsky Taxol total synthesis
Combined they provide a good insight in the application of organic chemistry in total synthesis.
The final step, the tail addition is identical to that of Nicolaou and is based on Ojima chemistry.
In terms of raw material shopping, this taxol molecule consists of the aforementioned Wieland-Miescher ketone, 2-methyl-3-pentanone, lithium aluminium hydride, osmium tetroxide, phenyllithium, pyridinium chlorochromate, the Corey-Chaykovsky reagent and acryloyl chloride.
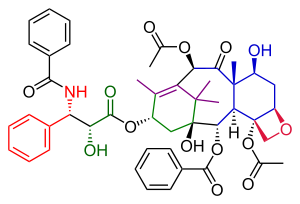
Taxol resulted from the tail addition of the Ojima lactam to alcohol 51, which is baccatin III (the original target molecule of the Danishefsky synthesis).
Two more hydroxyl groups were added by oxidation of the newly formed double bond with a catalytic amount of osmium tetroxide in the presence of N-methylmorpholine N-oxide.
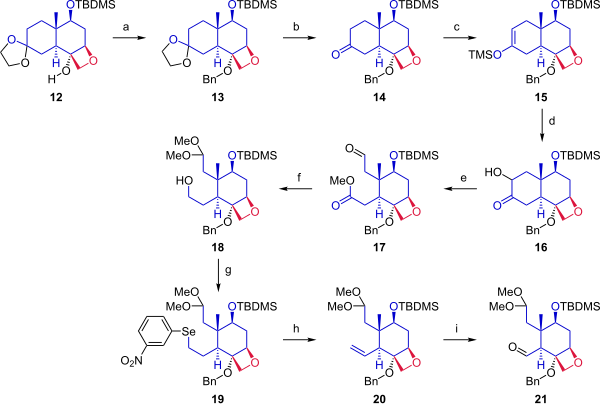
Heating this trimethylsilyl protected triflate in refluxing ethlyene glycol closed the ring to give oxetane 12.
In the next step, the aldehyde was protected as a dimethyl acetal, and the ester was reduced to give primary alcohol 18.
The hydroxyl group was converted in a Grieco elimination to the selenide (19), which on oxidation with hydrogen peroxide gave alkene 20.
For this synthesis (Scheme 3) the morpholine enamine of ethyl isopropyl ketone was treated with acryloyl chloride to give after hydrolysis diketone 25.
The intramolecular Heck reaction involved tetrakis(triphenylphosphine)palladium(0) and potassium carbonate in acetonitrile at reflux to give diene 39 and to complete the formation of the B ring.
The cleavage of the exocyclic double bond was difficult and accomplished only with forcing conditions (19 equivalents of osmium tetroxide, 105 °C, 24 hours) by the putative osmate ester (45).
The epoxide protecting group was removed with samarium (II) iodide[2] to give α-ß-unsaturated ketone 47.
The A ring was functionalized with a hydroxyl group through pyridinium chlorochromate oxidation of α-acylketone 49 to form ketone 50.