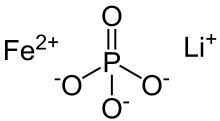
Lithium iron phosphate
Lithium iron phosphate exists naturally in the form of the mineral triphylite, but this material has insufficient purity for use in batteries.
The olivine structures of lithium rechargeable batteries are significant, for they are affordable, stable, and can be safely used to store energy.
[8] Arumugam Manthiram and John B. Goodenough first identified the polyanion class of cathode materials for lithium ion batteries.
Neutron diffraction confirmed that LFP was able to ensure the security of large input/output current of lithium batteries.
[14] The material can be produced by heating a variety of iron and lithium salts with phosphates or phosphoric acid.
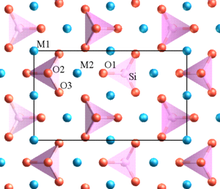
In crystallography, this structure is thought to belong to the Pmnb space group of the orthorhombic crystal system.
In contrast to two traditional cathode materials, LiMnO4 and LiCoO2, lithium ions of LiFePO4 migrate in the lattice's one-dimensional free volume.
FePO4 adopts a Pmnb space group with a unit cell volume of 272.4 Å3, only slightly smaller than that of its lithiated precursor.
LiFePO4's corner-shared FeO6 octahedra are separated by the oxygen atoms of the PO3−4 tetrahedra and cannot form a continuous FeO6 network, reducing conductivity.
A nearly close-packed hexagonal array of oxides centers provides relatively little free volume for Li+ ions to migrate within.
[18] LFP's major commercial advantages are that it poses few safety concerns such as overheating and explosion, as well as long cycle lifetimes, high power density and has a wider operating temperature range.
[19][20] BAE has announced that their HybriDrive Orion 7 hybrid bus uses about 180 kW LFP battery cells.
AES has developed multi-trillion watt battery systems that are capable of subsidiary services of the power network, including spare capacity and frequency adjustment.
The patent claims involved a unique crystal structure and a chemical formula of the battery cathode material.
In a parallel court proceeding, UT sued Valence Technology, a company that commercializes LFP products that alleged infringement.
After a Markman hearing, on April 27, 2011, the Western District Court of Texas held that the claims of the reexamined patents had a narrower scope than as originally granted.
In October 2008,[25] NTT announced that they would settle the case in the Japan Supreme Civil Court for $30 million.
NTT’s patent is also for an olivine LFP, with the general chemical formula of AyMPO4 (A is for alkali metal and M for the combination of Co and Fe), now used by BYD Company.
Similarly, LiMPO4 with an inorganic coating such as ZnO[28] and ZrO2,[29] has a better cycling lifetime, larger capacity and better characteristics under rapid discharge.
Mitsui Zosen and Aleees reported that addition of conducting metal particles such as copper and silver increased efficiency.
[31] Cyclic voltammetry confirms that LiFe1−xMxPO4, after metal substitution, has higher reversibility of lithium ion insertion and extraction.
[37] This capacity fade is primarily due to the solid electrolyte interface (SEI) formation reaction being accelerated by increasing temperature.
LFP batteries are especially affected by decreasing temperature which possibly hamper their application in high-latitude areas.
These losses are accounted for by the slow diffusion of lithium ions within electrodes and the formation of SEI that come with lower temperatures which subsequently increase the charge-transfer resistance on the electrolyte-electrode interfaces.
The colder condition leads to higher growth rates and shifts the initial point to lower state of charge which means that the plating process starts earlier.
The aggregated lithium ions are deposited on the surface of electrodes in the form of “plates” or even dendrites which may penetrate the separators, short-circuiting the battery completely.