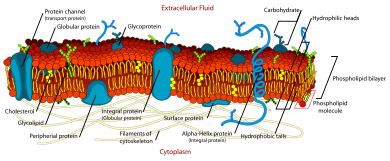
Fluid mosaic model
The biological model, which was devised by Seymour Jonathan Singer and Garth L. Nicolson in 1972,[1] describes the cell membrane as a two-dimensional liquid where embedded proteins are generally randomly distributed.
For example, it is stated that "A prediction of the fluid mosaic model is that the two-dimensional long-range distribution of any integral protein in the plane of the membrane is essentially random.
The fluid property of functional biological membranes had been determined through labeling experiments, x-ray diffraction, and calorimetry.
[2] An important experiment that provided evidence supporting fluid and dynamic biological was performed by Frye and Edidin.
Using antibody staining, they were able to show that the mouse and human proteins remained segregated to separate halves of the heterokaryon a short time after cell fusion.
Lowering the temperature slowed the rate of this diffusion by causing the membrane phospholipids to transition from a fluid to a gel phase.
While Singer and Nicolson had substantial evidence drawn from multiple subfields to support their model, recent advances in fluorescence microscopy and structural biology have validated the fluid mosaic nature of cell membranes.
[4] Another form of asymmetry was shown by the work of Mouritsen and Bloom in 1984, where they proposed a Mattress Model of lipid-protein interactions to address the biophysical evidence that the membrane can range in thickness and hydrophobicity of proteins.
[5] The existence of non-bilayer lipid formations with important biological functions was confirmed subsequent to publication of the fluid mosaic model.
Moreover, they impose physical constraints that restrict the free lateral diffusion of proteins and at least some lipids within the bilipid layer.
[13] Both processes restrict the diffusion of proteins and lipids directly involved, as well as of other interacting components of the cell membranes.


Septin ring-like structures (in green) can pinch cell membranes and split them into subdomains.