MXenes
In materials science, MXenes are a class of two-dimensional inorganic compounds along with MBenes, that consist of atomically thin layers of transition metal carbides, nitrides, or carbonitrides.
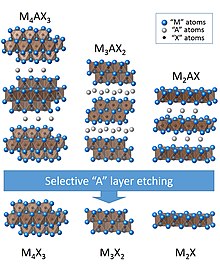
[6] For solid-solution MXenes, they have the general formulas: (M'2−yM"y)C, (M'3−yM"y)C2, (M'4−yM"y)C3, or (M'5−yM"y)C4, where the metals are randomly distributed throughout the structure in solid solutions leading to continuously tailorable properties.
[16] A general Lewis acid molten salt approach was proven viable to etch most of MAX phases members (such as MAX-phase precursors with A elements Si, Zn, and Ga) by some other melts (CdCl2, FeCl2, CoCl2, CuCl2, AgCl, and NiCl2).
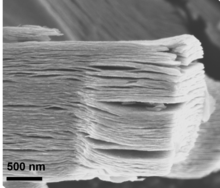
This procedure etches out Al, yielding multilayered Ti4N3, which can further be delaminated into single and few layers by immersing the MXene in tetrabutylammonium hydroxide, followed by sonication.
[19] Recently, single crystalline monolayer W5N6 has been successfully synthesized by CVD in wafer scale[20][21] which shows promise of MXenes in electronic application in the future.
[22] In this etching method, the MAX phase is treated in the solution of acid and salt under high pressure and temperature conditions.
[38] The strategy involves installation and removal of the surface groups by performing substitution and elimination reactions in molten inorganic salts.
[43] For the case of Ti3C2Tx and Ti2CTx, etching with concentrated hydrofluoric acid leads to open, accordion-like morphology with a compact distance between layers (this is common for other MXene compositions as well).
However, when etching is conducted with hydrochloric acid and LiF as a fluoride source, morphology is more compact with a larger inter-layer spacing, presumably due to amounts of intercalated water.
This can be achieved by Minimally Intensive Layer Delamination (MILD) method, where the quantity of LiF to MAX phase is scaled up resulting in flakes that can be delminated in situ when washing to neutral pH.
[62] For the wavelengths above 1.4 micrometer, these materials show negative permittivity, resulting in a strong metallic response to the IR light.
[62] Such materials that are visible black but IR white are highly desired in many areas, such as camouflage, thermal management, and information encryption.
[65] Colony forming unit and regrowth curves showed that more than 98% of both bacterial cells lost viability at 200 μg/mL Ti3C2 colloidal solution within 4 h of exposure.
It was shown that Ti3C2 MXene may affect the occurrence of oxidative stress and, in consequence, the generation of reactive oxygen species (ROS).
[67] Recently, Ti3C2 MXenes have been used as flowing electrodes in a flow-electrode capacitive deionization cell for the removal of ammonia from simulated wastewater.
MXene FE-CDI demonstrated a 100x improvement in ion absorption capacity at 10x greater energy efficiency as compared to activated carbon flowing electrodes.
[68] One-micron-thick Ti3C2 MXene membranes demonstrated ultrafast water flux (approximately 38 L/(Bar·h·m2)) and differential sieving of salts depending on both the hydration radius and charge of the ions.
[69] As conductive layered materials with tunable surface terminations, MXenes have been shown to be promising for energy storage applications (Li-ion batteries, supercapacitors, and energy storage components),[70][71] composites, photocatalysis,[72] water purification,[69] gas sensors,[73][74] transparent conducting electrodes,[47] neural electrodes,[67] as a metamaterial,[75] SERS substrate,[76] photonic diode,[77] electrochromic device,[48] and triboelectric nanogenerator (TENGs).
By virtue of higher electrochemically active and accessible surface area, delaminated Ti3C2Tx paper demonstrates a reversible capacity of 410 mAhg−1 at 1C and 110 mAhg−1 at 36C rate.
Moreover, the experimentally measured capacity for Ti3C2Tx paper is higher than predicted from computer simulations, indicating that further investigation is required to ascertain the charge storage mechanism.
[83][84] As a typical example, multilayered Ti2CTx MXene as a negative electrode material showed a capacity of 175 mA h g−1 and good rate capability.
[86] Porous MXene-based paper electrodes have been reported to exhibit high volumetric capacities and stable cycling performance, demonstrating promise for devices where size matters.
The synthesis route controls the surface chemistry and plays a large role in determining the intercalation reaction rate and the charge storage density.
[93] Supercapacitor electrodes based on Ti3C2Tx MXene paper in aqueous solutions demonstrate excellent cyclability and the ability to store 300-400 F/cm3, which translates to three times as much energy as for activated carbon and graphene-based capacitors.
[12] In Ti3C2Tx MXene electrodes for lithium-ion electrolytes, the choice of solvent greatly affected the ion transport and intercalation kinetics.
[95] FL-Ti3C2 (the most studied MXene) nanosheets can mix intimately with polymers such as polyvinyl alcohol (PVA), forming alternating MXene-PVA layered structures.
[105] Porous Ti3C2 has a larger specific surface area and more open structure, and can be filtered as flexible films with, or without, the addition of carbon nanotubes (CNTs).
[105] The as-fabricated p-Ti3C2/CNT films showed significantly improved lithium ion storage capabilities, with a capacity as high as 1250 mA·h·g−1 at 0.1 C, excellent cycling stability, and good rate performance.
[106][107] MXene SERS substrates have been manufactured by spray-coating and were used to detect several common dyes, with calculated enhancement factors reaching ~106.
[76] Transparent conducting electrodes have been fabricated with titanium carbide MXene showing the ability to transmit approximately 97% of visible light per nanometer thickness.