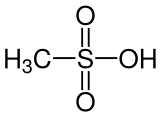
Methanesulfonic acid
[3] German chemist Hermann Kolbe discovered MSA between 1842 and 1845 and originally termed it methyl hyposulphuric acid.
[4][5] CCl3SO3H + 3 H → CHCl2SO3H + 2 H + HCl → … → CH3SO3H + 3 HCl Kolbe's research on methanesulfonic and chloroacetic acids was hailed by Berzelius as strong evidence for his theory of copulated compounds, a modification of radical theory to accommodate substitution reactions which posited the combination of organic and inorganic moieties without significantly altering the properties of the latter.
Other historical laboratory synthesis routes included oxidizing methanethiol, dimethyl disulfide or methyl thiocyanate with nitric acid.
Between years 1970 and 2000 MSA was used only on a relatively small-scale in niche markets (for example, in the microelectronic and electroplating industries since the 1980s), which was mainly due to its rather high price and limited availability.
However, this situation changed around 2003, when BASF launched commercial production of MSA in Ludwigshafen based on a modified version of the aforementioned air oxidation process, oxidising dimethyldisulfide with nitric acid which is then restored using atmospheric oxygen.
[9] An even better (lower-cost and environmentally friendlier) process of making methanesulfonic acid was developed in 2016 by Grillo-Werke AG (Germany).