Myristoylation
[2] This lipidation event is the most common type of fatty acylation [3] and is present in many organisms, including animals, plants, fungi, protozoans [4] and viruses.
In 1982, Koiti Titani's lab identified an "N-terminal blocking group" on the catalytic subunit of cyclic AMP-dependent protein kinase in cows as n-tetradecanoyl.
[6] Almost simultaneously in Claude B. Klee's lab, this same N-terminal blocking group was further characterized as myristic acid.
[7] Both labs made this discovery utilizing similar techniques: mass spectrometry and gas chromatography.
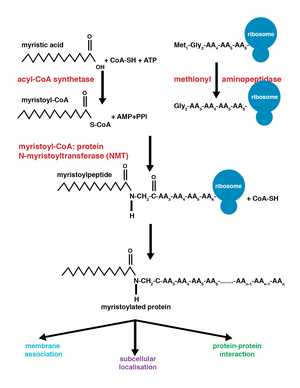
[6][7] The enzyme N-myristoyltransferase (NMT) or glycylpeptide N-tetradecanoyltransferase is responsible for the irreversible addition of a myristoyl group to N-terminal or internal glycine residues of proteins.
First, myristoyl coenzyme A (CoA) is positioned in its binding pocket of NMT so that the carbonyl faces two amino acid residues, phenylalanine 170 and leucine 171.
[9] This polarizes the carbonyl so that there is a net positive charge on the carbon, making it susceptible to nucleophilic attack by the glycine residue of the protein to be modified.
[2] Co-translational and post-translational covalent modifications enable proteins to develop higher levels of complexity in cellular function, further adding diversity to the proteome.
[1] Post-translational myristoylation typically occurs following a caspase cleavage event, resulting in the exposure of an internal glycine residue, which is then available for myristic acid addition.
"[13] Both hydrophobic myristoyl groups and "basic patches" (highly positive regions on the protein) characterize myristoyl-electrostatic switches.
The basic patch allows for favorable electrostatic interactions to occur between the negatively charged phospholipid heads of the membrane and the positive surface of the associating protein.
[5] Myristoylation plays a vital role in membrane targeting and signal transduction[16] in plant responses to environmental stress.
[8] Actin, gelsolin and p21-activated kinase 2 PAK2 are three other proteins that are myristoylated following cleavage by caspase 3, which leads to either the up-regulation or down-regulation of apoptosis.
[8] c-Src is a gene that codes for proto-oncogene tyrosine-protein kinase Src, a protein important for normal mitotic cycling.
[19] HIV-1 is a retrovirus that relies on myristoylation of one of its structural proteins in order to successfully package its genome, assemble and mature into a new infectious particle.
In addition to prokaryotic bacteria, the NMTs of numerous disease-causing eukaryotic organisms have been identified as drug targets as well.