Single-domain antibody
[4] Single-domain antibodies are being researched for multiple pharmaceutical applications, and have potential for use in the treatment of acute coronary syndrome, cancer, Alzheimer's disease,[5][6] and Covid-19.
Large phage displayed VNAR and VHH single domain libraries were established from nurse sharks[17] and dromedary camels.
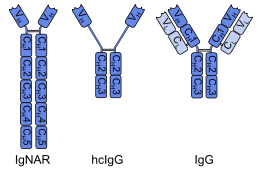
A problem with this approach is that the binding region of common IgG consists of two domains (VH and VL), which tend to dimerize or aggregate because of their lipophilicity.
The HN3 human single-domain antibodies have been used to create immunotoxins [31][32][33] and chimeric antigen receptor (CAR) T cells[34] for treating liver cancer.
[35] Single-domain antibodies allow a broad range of applications in biotechnical as well as therapeutic use due to their small size, simple production and high affinity.
[38] The coupling of an anti-GFP Nanobody to a monovalent matrix, called GFP-nanotrap, allows the isolation of GFP-fusion proteins and their interacting partners for further biochemical analyses.
[39] Single molecule localization with super-resolution imaging techniques requires the specific delivery of fluorophores into close proximity with a target protein.
Due to their large size the use of antibodies coupled to organic dyes can often lead to a misleading signal owing to the distance between the fluorophore and the target protein.
The fusion of organic dyes to anti-GFP Nanobodies targeting GFP-tagged proteins allows nanometer spatial resolution and minimal linkage error because of the small size and high affinity.
In addition to their advantage in targeting less accessible epitopes, their conformational stability also leads to higher resistance to surface regeneration conditions.
[10] As an approach for photothermal therapy Nanobodies binding to the HER2 antigen, which is overexpressed in breast and ovarian cancer cells, were conjugated to branched gold nanoparticles (see figure).
[49] Caplacizumab, a single-domain antibody targeting von Willebrand factor is in clinical trials for the prevention of thrombosis in patients with acute coronary syndrome.
[51] Ablynx expects that their Nanobodies might cross the blood–brain barrier and permeate into large solid tumours more easily than whole antibodies, which would allow for the development of drugs against brain cancers.
Stijlemans et al. 2004 succeeded in inducing effective sdAbs from rabbit and Camelus dromedarius by displaying a variable surface glycoprotein antigen to the vertebrates' immune systems using a phage.

The extended CDR3 loop is coloured orange.